Proteomics of Urinary Vesicles Links Plakins and Complement to Polycystic Kidney Disease
- PMID: 26940098
- PMCID: PMC5042669
- DOI: 10.1681/ASN.2015090994
Proteomics of Urinary Vesicles Links Plakins and Complement to Polycystic Kidney Disease
Abstract
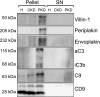
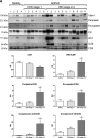
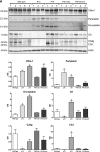
Novel therapies in autosomal dominant polycystic kidney disease (ADPKD) signal the need for markers of disease progression or response to therapy. This study aimed to identify disease-associated proteins in urinary extracellular vesicles (uEVs), which include exosomes, in patients with ADPKD. We performed quantitative proteomics on uEVs from healthy controls and patients with ADPKD using a labeled approach and then used a label-free approach with uEVs of different subjects (healthy controls versus patients with ADPKD versus patients with non-ADPKD CKD). In both experiments, 30 proteins were consistently more abundant (by two-fold or greater) in ADPKD-uEVs than in healthy- and CKD-uEVs. Of these proteins, we selected periplakin, envoplakin, villin-1, and complement C3 and C9 for confirmation because they were also significantly overrepresented in pathway analysis and were previously implicated in ADPKD pathogenesis. Immunoblotting confirmed higher abundances of the selected proteins in uEVs from three independent groups of patients with ADPKD. Whereas uEVs of young patients with ADPKD and preserved kidney function already had higher levels of complement, only uEVs of patients with advanced stages of ADPKD had increased levels of villin-1, periplakin, and envoplakin. Furthermore, all five proteins correlated positively with total kidney volume. Analysis in kidney tissue from mice with kidney-specific, tamoxifen-inducible Pkd1 deletion demonstrated higher expression in more severe stages of the disease and correlation with kidney weight for each protein of interest. In summary, proteomic analysis of uEVs identified plakins and complement as disease-associated proteins in ADPKD. These proteins are new candidates for evaluation as biomarkers or targets for therapy in ADPKD.
Keywords: ADPKD; complement; cytoskeleton; genetic renal disease.
Copyright © 2016 by the American Society of Nephrology.
Figures






References
-
- Neumann HP, Jilg C, Bacher J, Nabulsi Z, Malinoc A, Hummel B, Hoffmann MM, Ortiz-Bruechle N, Glasker S, Pisarski P, Neeff H, Krämer-Guth A, Cybulla M, Hornberger M, Wilpert J, Funk L, Baumert J, Paatz D, Baumann D, Lahl M, Felten H, Hausberg M, Zerres K, Eng C Else-Kroener-Fresenius-ADPKD-Registry : Epidemiology of autosomal-dominant polycystic kidney disease: An in-depth clinical study for south-western Germany. Nephrol Dial Transplant 28: 1472–1487, 2013 - PubMed
-
- Ong AC, Devuyst O, Knebelmann B, Walz G ERA-EDTA Working Group for Inherited Kidney Diseases : Autosomal dominant polycystic kidney disease: The changing face of clinical management. Lancet 385: 1993–2002, 2015 - PubMed
-
- Dear JW, Street JM, Bailey MA: Urinary exosomes: A reservoir for biomarker discovery and potential mediators of intrarenal signalling. Proteomics 13: 1572–1580, 2013 - PubMed
-
- Salih M, Zietse R, Hoorn EJ: Urinary extracellular vesicles and the kidney: Biomarkers and beyond. Am J Physiol Renal Physiol 306: F1251–F1259, 2014 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

