Point-of-care C reactive protein for the diagnosis of lower respiratory tract infection in NHS primary care: a qualitative study of barriers and facilitators to adoption
- PMID: 26940107
- PMCID: PMC4785316
- DOI: 10.1136/bmjopen-2015-009959
Point-of-care C reactive protein for the diagnosis of lower respiratory tract infection in NHS primary care: a qualitative study of barriers and facilitators to adoption
Abstract
Objectives: Point-of-care (POC) C reactive protein (CRP) is incorporated in National Institute of Health and Care Excellence (NICE) guidelines for the diagnosis of pneumonia, reduces antibiotic prescribing and is cost effective.
Aim: To determine the barriers and facilitators to adoption of POC CRP testing in National Health Service (NHS) primary care for the diagnosis of lower respiratory tract infection.
Design: The study followed a qualitative methodology based on grounded theory. The study was undertaken in 2 stages. Stage 1 consisted of semistructured interviews with 8 clinicians from Europe and the UK who use the test in routine practice, and focused on their subjective experience in the challenges of implementing POC CRP testing. Stage 2 was a multidisciplinary-facilitated workshop with NHS stakeholders to discuss barriers to adoption, impact of adoption and potential adoption scenarios. Emergent theme analysis was undertaken.
Participants: Participants included general practitioners (including those with commissioning experience), biochemists, pharmacists, clinical laboratory scientists and industry representatives from the UK and abroad.
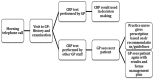
Results: Barriers to the implementation of POC CRP exist, but successful adoption has been demonstrated abroad. Analysis highlighted 7 themes: reimbursement and incentivisation, quality control and training, laboratory services, practitioner attitudes and experiences, effects on clinic flow and workload, use in pharmacy and gaps in evidence.
Conclusions: Successful adoption models from the UK and abroad demonstrate a distinctive pattern and involve collaboration with central laboratory services. Incorporating antimicrobial stewardship into quality improvement frameworks may incentivise adoption. Further research is needed to develop scaling-up strategies to address the resourcing, clinical governance and economic impact of widespread NHS implementation.
Keywords: PRIMARY CARE; QUALITATIVE RESEARCH.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/
Figures
Similar articles
-
Qualitative study to explore the views of general practice staff on the use of point-of-care C reactive protein testing for the management of lower respiratory tract infections in routine general practice in England.BMJ Open. 2018 Oct 24;8(10):e023925. doi: 10.1136/bmjopen-2018-023925. BMJ Open. 2018. PMID: 30361406 Free PMC article. Clinical Trial.
-
Funding and policy incentives to encourage implementation of point-of-care C-reactive protein testing for lower respiratory tract infection in NHS primary care: a mixed-methods evaluation.BMJ Open. 2018 Oct 25;8(10):e024558. doi: 10.1136/bmjopen-2018-024558. BMJ Open. 2018. PMID: 30366918 Free PMC article.
-
Qualitative study of primary care clinicians' views on point-of-care testing for C-reactive protein for acute respiratory tract infections in family medicine.BMJ Open. 2017 Jan 25;7(1):e012503. doi: 10.1136/bmjopen-2016-012503. BMJ Open. 2017. PMID: 28122829 Free PMC article.
-
Adoption of C-reactive protein point-of-care tests for the management of acute childhood infections in primary care in the Netherlands and England: a comparative health systems analysis.BMC Health Serv Res. 2023 Feb 23;23(1):191. doi: 10.1186/s12913-023-09065-8. BMC Health Serv Res. 2023. PMID: 36823597 Free PMC article. Review.
-
Respiratory tract infections (RTIs) in primary care: narrative review of C reactive protein (CRP) point-of-care testing (POCT) and antibacterial use in patients who present with symptoms of RTI.BMJ Open Respir Res. 2020 Sep;7(1):e000624. doi: 10.1136/bmjresp-2020-000624. BMJ Open Respir Res. 2020. PMID: 32895246 Free PMC article. Review.
Cited by
-
Qualitative study to explore the views of general practice staff on the use of point-of-care C reactive protein testing for the management of lower respiratory tract infections in routine general practice in England.BMJ Open. 2018 Oct 24;8(10):e023925. doi: 10.1136/bmjopen-2018-023925. BMJ Open. 2018. PMID: 30361406 Free PMC article. Clinical Trial.
-
Qualitative analysis of stakeholder interviews to identify the barriers and facilitators to the adoption of point-of-care diagnostic tests in the UK.BMJ Open. 2021 Apr 13;11(4):e042944. doi: 10.1136/bmjopen-2020-042944. BMJ Open. 2021. PMID: 33849848 Free PMC article.
-
The social role of C-reactive protein point-of-care testing to guide antibiotic prescription in Northern Thailand.Soc Sci Med. 2018 Apr;202:1-12. doi: 10.1016/j.socscimed.2018.02.018. Epub 2018 Feb 23. Soc Sci Med. 2018. PMID: 29499522 Free PMC article. Clinical Trial.
-
Implementing interventions to reduce antibiotic use: a qualitative study in high-prescribing practices.BMC Fam Pract. 2021 Jan 23;22(1):25. doi: 10.1186/s12875-021-01371-6. BMC Fam Pract. 2021. PMID: 33485324 Free PMC article.
-
Point-of-care tests to manage acute respiratory tract infections in primary care: a systematic review and qualitative synthesis of healthcare professional and patient views.J Antimicrob Chemother. 2025 Jan 3;80(1):29-46. doi: 10.1093/jac/dkae349. J Antimicrob Chemother. 2025. PMID: 39378128 Free PMC article.
References
-
- Verlee L, Verheij TJ, Hopstaken RM et al. . [Summary of NHG practice guideline ‘Acute cough’]. Ned Tijdschr Geneeskd 2012;156:A4188. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous