Protein Partners of α-Synuclein in Health and Disease
- PMID: 26940507
- PMCID: PMC8029326
- DOI: 10.1111/bpa.12374
Protein Partners of α-Synuclein in Health and Disease
Abstract
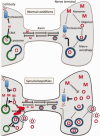
α-synuclein is normally situated in the nerve terminal but it accumulates and aggregates in axons and cell bodies in synucleinopathies such as Parkinson's disease. The conformational changes occurring during α-synucleins aggregation process affects its interactions with other proteins and its subcellular localization. This review focuses on interaction partners of α-synuclein within different compartments of the cell with a focus on those preferentially binding aggregated α-synuclein. The aggregation state of α-synuclein also affects its catabolism and we hypothesize impaired macroautophagy is involved neuronal excretion of α-synuclein species responsible for the prion-like spreading of α-synuclein pathology.
Keywords: Lewy Body; Parkinson's disease; dementia; neurodegeneration; protein interaction; proteomics; α-synuclein.
© 2016 International Society of Neuropathology.
Figures
Similar articles
-
p25alpha Stimulates alpha-synuclein aggregation and is co-localized with aggregated alpha-synuclein in alpha-synucleinopathies.J Biol Chem. 2005 Feb 18;280(7):5703-15. doi: 10.1074/jbc.M410409200. Epub 2004 Dec 7. J Biol Chem. 2005. PMID: 15590652
-
The interaction of α-synuclein and Tau: A molecular conspiracy in neurodegeneration?Semin Cell Dev Biol. 2020 Mar;99:55-64. doi: 10.1016/j.semcdb.2018.05.005. Epub 2018 May 10. Semin Cell Dev Biol. 2020. PMID: 29738880 Review.
-
The Role of α-Synuclein in Etiology of Neurodegenerative Diseases.Int J Mol Sci. 2024 Aug 24;25(17):9197. doi: 10.3390/ijms25179197. Int J Mol Sci. 2024. PMID: 39273146 Free PMC article. Review.
-
ɑ-Synuclein strains and the variable pathologies of synucleinopathies.J Neurochem. 2016 Oct;139 Suppl 1:256-274. doi: 10.1111/jnc.13595. Epub 2016 Mar 30. J Neurochem. 2016. PMID: 26924014 Review.
-
Prion-like propagation of α-synuclein in neurodegenerative diseases.Prog Mol Biol Transl Sci. 2019;168:323-348. doi: 10.1016/bs.pmbts.2019.07.005. Epub 2019 Jul 31. Prog Mol Biol Transl Sci. 2019. PMID: 31699325 Review.
Cited by
-
Alpha-Synuclein Modulates the Physical Properties of DNA.Chemistry. 2018 Oct 17;24(58):15685-15690. doi: 10.1002/chem.201803933. Epub 2018 Sep 19. Chemistry. 2018. PMID: 30102440 Free PMC article.
-
pH-Dependent Aggregation in Intrinsically Disordered Proteins Is Determined by Charge and Lipophilicity.Cells. 2020 Jan 8;9(1):145. doi: 10.3390/cells9010145. Cells. 2020. PMID: 31936201 Free PMC article.
-
Navigating the dynamic landscape of alpha-synuclein morphology: a review of the physiologically relevant tetrameric conformation.Neural Regen Res. 2020 Mar;15(3):407-415. doi: 10.4103/1673-5374.265792. Neural Regen Res. 2020. PMID: 31571649 Free PMC article. Review.
-
Investigation of α-Synuclein Amyloid Fibrils Using the Fluorescent Probe Thioflavin T.Int J Mol Sci. 2018 Aug 23;19(9):2486. doi: 10.3390/ijms19092486. Int J Mol Sci. 2018. PMID: 30142878 Free PMC article.
-
Recent advances on the molecular pathogenesis of prion diseases.Brain Pathol. 2019 Mar;29(2):245-247. doi: 10.1111/bpa.12693. Brain Pathol. 2019. PMID: 30588674 Free PMC article. No abstract available.
References
-
- Baek JH, Whitfield D, Howlett D, Francis P, Bereczki E, Ballard C et al (2015) Unfolded protein response is activated in Lewy body dementias. Neuropathol Appl Neurobiol [Epub ahead of print; doi: 10.1111/nan.12260]. - PubMed
-
- Bellucci A, Navarria L, Zaltieri M, Falarti E, Bodei S, Sigala S et al (2011) Induction of the unfolded protein response by alpha‐synuclein in experimental models of Parkinson's disease. J Neurochem 116:588–605. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

