Nicorandil, a Nitric Oxide Donor and ATP-Sensitive Potassium Channel Opener, Protects Against Dystrophin-Deficient Cardiomyopathy
- PMID: 26940570
- PMCID: PMC5010518
- DOI: 10.1177/1074248416636477
Nicorandil, a Nitric Oxide Donor and ATP-Sensitive Potassium Channel Opener, Protects Against Dystrophin-Deficient Cardiomyopathy
Abstract
Background: Dystrophin-deficient cardiomyopathy is a growing clinical problem without targeted treatments. We investigated whether nicorandil promotes cardioprotection in human dystrophin-deficient induced pluripotent stem cell (iPSC)-derived cardiomyocytes and the muscular dystrophy mdx mouse heart.
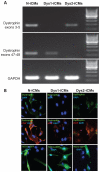
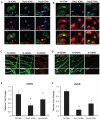
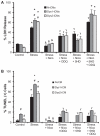
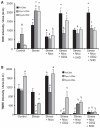
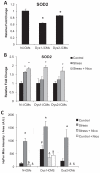
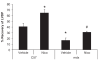
Methods and results: Dystrophin-deficient iPSC-derived cardiomyocytes had decreased levels of endothelial nitric oxide synthase and neuronal nitric oxide synthase. The dystrophin-deficient cardiomyocytes had increased cell injury and death after 2 hours of stress and recovery. This was associated with increased levels of reactive oxygen species and dissipation of the mitochondrial membrane potential. Nicorandil pretreatment was able to abolish these stress-induced changes through a mechanism that involved the nitric oxide-cyclic guanosine monophosphate pathway and mitochondrial adenosine triphosphate-sensitive potassium channels. The increased reactive oxygen species levels in the dystrophin-deficient cardiomyocytes were associated with diminished expression of select antioxidant genes and increased activity of xanthine oxidase. Furthermore, nicorandil was found to improve the restoration of cardiac function after ischemia and reperfusion in the isolated mdx mouse heart.
Conclusion: Nicorandil protects against stress-induced cell death in dystrophin-deficient cardiomyocytes and preserves cardiac function in the mdx mouse heart subjected to ischemia and reperfusion injury. This suggests a potential therapeutic role for nicorandil in dystrophin-deficient cardiomyopathy.
Keywords: cardiomyopathy; induced pluripotent cells; muscular dystrophy; nicorandil.
© The Author(s) 2016.
Figures


References
-
- Birnkrant DJ, Ararat E, Mhanna MJ. Cardiac phenotype determines survival in Duchenne muscular dystrophy. Pediatr Pulmonol. 2016;51(1):70–76. - PubMed
-
- Jung C, Martins AS, Niggli E, Shirokova N. Dystrophic cardiomyopathy: amplification of cellular damage by Ca2+ signalling and reactive oxygen species-generating pathways. Cardiovasc Res. 2008;77(4):766–773. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

