Sequential psychological and pharmacological therapies for comorbid and primary insomnia: study protocol for a randomized controlled trial
- PMID: 26940892
- PMCID: PMC4778294
- DOI: 10.1186/s13063-016-1242-3
Sequential psychological and pharmacological therapies for comorbid and primary insomnia: study protocol for a randomized controlled trial
Abstract
Background: Chronic insomnia is a prevalent disorder associated with significant psychosocial, health, and economic impacts. Cognitive behavioral therapies (CBTs) and benzodiazepine receptor agonist (BzRA) medications are the most widely supported therapeutic approaches for insomnia management. However, few investigations have directly compared their relative and combined benefits, and even fewer have tested the benefits of sequential treatment for those who do not respond to initial insomnia therapy. Moreover, insomnia treatment studies have been limited by small, highly screened study samples, fixed-dose, and fixed-agent pharmacotherapy strategies that do not represent usual clinical practices. This study will address these limitations.
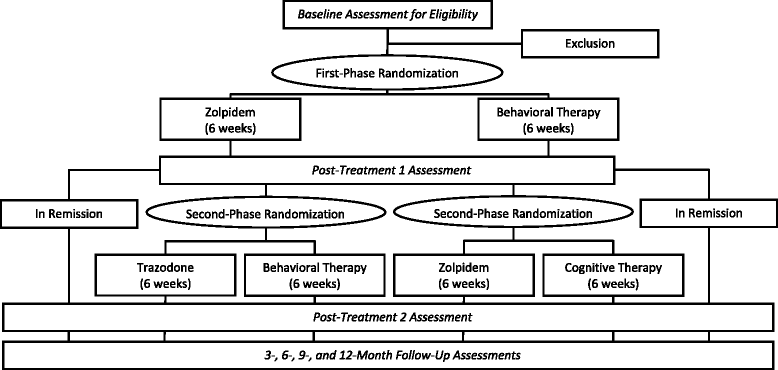
Methods/design: This is a two-site randomized controlled trial, which will enroll 224 adults who meet the criteria for a chronic insomnia disorder with or without comorbid psychiatric disorders. Prospective participants will complete clinical assessments and polysomnography and then will be randomly assigned to first-stage therapy involving either behavioral therapy (BT) or zolpidem. Treatment outcomes will be assessed after 6 weeks, and treatment remitters will be followed for the next 12 months on maintenance therapy. Those not achieving remission will be offered randomization to a second, 6-week treatment, again involving either pharmacotherapy (zolpidem or trazodone) or psychological therapy (BT or cognitive therapy (CT)). All participants will be re-evaluated 12 weeks after the protocol initiation and at 3-, 6-, 9-, and 12-month follow-ups. Insomnia remission, defined categorically as a score < 8 on the Insomnia Severity Index, a patient-reported outcome, will serve as the primary endpoint for treatment comparisons. Secondary outcomes will include sleep parameters derived from daily sleep diaries and from polysomnography, subjective measures of fatigue, mood, quality of life, and functional impairments; and measures of adverse events; dropout rates; and treatment acceptability. Centrally trained therapists will administer therapies according to manualized, albeit flexible, treatment algorithms.
Discussion: This clinical trial will provide new information about optimal treatment sequencing and will have direct implication for the development of clinical guidelines for managing chronic insomnia with and without comorbid psychiatric conditions.
Trial registration: ClinicalTrials.gov Identifier: NCT01651442 , Protocol version 4, 20 April 2011, registered 26 June 2012.
Similar articles
-
Effect of Psychological and Medication Therapies for Insomnia on Daytime Functions: A Randomized Clinical Trial.JAMA Netw Open. 2023 Dec 1;6(12):e2349638. doi: 10.1001/jamanetworkopen.2023.49638. JAMA Netw Open. 2023. PMID: 38153735 Free PMC article. Clinical Trial.
-
Effectiveness of Sequential Psychological and Medication Therapies for Insomnia Disorder: A Randomized Clinical Trial.JAMA Psychiatry. 2020 Nov 1;77(11):1107-1115. doi: 10.1001/jamapsychiatry.2020.1767. JAMA Psychiatry. 2020. PMID: 32639561 Free PMC article. Clinical Trial.
-
Cognitive behavioral therapy, singly and combined with medication, for persistent insomnia: a randomized controlled trial.JAMA. 2009 May 20;301(19):2005-15. doi: 10.1001/jama.2009.682. JAMA. 2009. PMID: 19454639 Free PMC article. Clinical Trial.
-
Sleep maintenance insomnia: strengths and weaknesses of current pharmacologic therapies.Ann Clin Psychiatry. 2006 Jan-Mar;18(1):49-56. doi: 10.1080/10401230500464711. Ann Clin Psychiatry. 2006. PMID: 16517453 Review.
-
Diagnosis and treatment of chronic insomnia: a review.Psychiatr Serv. 2005 Mar;56(3):332-43. doi: 10.1176/appi.ps.56.3.332. Psychiatr Serv. 2005. PMID: 15746509 Review.
Cited by
-
Stepped care management of insomnia co-occurring with sleep apnea: the AIR study protocol.Trials. 2022 Sep 24;23(1):806. doi: 10.1186/s13063-022-06753-4. Trials. 2022. PMID: 36153634 Free PMC article.
-
Group cognitive behavioural therapy for insomnia compared with treatment as usual for sleep problems in psychiatric care (the SIP trials): a protocol for a pragmatic, randomised controlled trial.BMJ Open. 2025 Apr 17;15(4):e090997. doi: 10.1136/bmjopen-2024-090997. BMJ Open. 2025. PMID: 40250874 Free PMC article.
-
Effect of Psychological and Medication Therapies for Insomnia on Daytime Functions: A Randomized Clinical Trial.JAMA Netw Open. 2023 Dec 1;6(12):e2349638. doi: 10.1001/jamanetworkopen.2023.49638. JAMA Netw Open. 2023. PMID: 38153735 Free PMC article. Clinical Trial.
-
Off-Label Medication: From a Simple Concept to Complex Practical Aspects.Int J Environ Res Public Health. 2021 Oct 4;18(19):10447. doi: 10.3390/ijerph181910447. Int J Environ Res Public Health. 2021. PMID: 34639747 Free PMC article. Review.
-
Trazodone for Insomnia: A Systematic Review.Innov Clin Neurosci. 2017 Aug 1;14(7-8):24-34. eCollection 2017 Jul-Aug. Innov Clin Neurosci. 2017. PMID: 29552421 Free PMC article. Review.
References
-
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5) 5. Washington, DC: American Psychiatric Publishing; 2013.
-
- Edinger JD, Bonnet MH, Bootzin RR, Doghramji K, Dorsey CM, Espie CA, et al. Derivation of research diagnostic criteria for insomnia: report of an American Academy of Sleep Medicine Work Group. Sleep. 2004;27:1567–96. - PubMed
-
- American Academy of Sleep Medicine . International Classification of Sleep Disorders: Diagnostic and Coding Manuel. 2. Westchester: American Academy of Sleep Medicine; 2005.
-
- American Academy of Sleep Medicine . International Classification of Sleep Disorders: Diagnostic and Coding Manuel, 3rd ed (ICSD-3) 3. Westchester: American Academy of Sleep Medicine; 2014.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous