Evidence for Mechanisms Underlying the Functional Benefits of a Myocardial Matrix Hydrogel for Post-MI Treatment
- PMID: 26940929
- PMCID: PMC4779189
- DOI: 10.1016/j.jacc.2015.12.035
Evidence for Mechanisms Underlying the Functional Benefits of a Myocardial Matrix Hydrogel for Post-MI Treatment
Abstract
Background: There is increasing need for better therapies to prevent the development of heart failure after myocardial infarction (MI). An injectable hydrogel derived from decellularized porcine ventricular myocardium has been shown to halt the post-infarction progression of negative left ventricular remodeling and decline in cardiac function in both small and large animal models.
Objectives: This study sought to elucidate the tissue-level mechanisms underlying the therapeutic benefits of myocardial matrix injection.
Methods: Myocardial matrix or saline was injected into infarcted myocardium 1 week after ischemia-reperfusion in Sprague-Dawley rats. Cardiac function was evaluated by magnetic resonance imaging and hemodynamic measurements at 5 weeks after injection. Whole transcriptome microarrays were performed on RNA isolated from the infarct at 3 days and 1 week after injection. Quantitative polymerase chain reaction and histologic quantification confirmed expression of key genes and their activation in altered pathways.
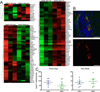
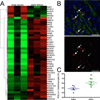
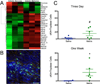
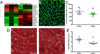
Results: Principal component analysis of the transcriptomes showed that samples collected from myocardial matrix-injected infarcts are distinct and cluster separately from saline-injected control subjects. Pathway analysis indicated that these differences are due to changes in several tissue processes that may contribute to improved cardiac healing after MI. Matrix-injected infarcted myocardium exhibits an altered inflammatory response, reduced cardiomyocyte apoptosis, enhanced infarct neovascularization, diminished cardiac hypertrophy and fibrosis, altered metabolic enzyme expression, increased cardiac transcription factor expression, and progenitor cell recruitment, along with improvements in global cardiac function and hemodynamics.
Conclusions: These results indicate that the myocardial matrix alters several key pathways after MI creating a pro-regenerative environment, further demonstrating its promise as a potential post-MI therapy.
Keywords: biomaterial; extracellular matrix; heart failure; infarction; microarray.
Copyright © 2016 American College of Cardiology Foundation. Published by Elsevier Inc. All rights reserved.
Figures
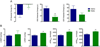



Comment in
-
Decellularized Scaffold Hydrogel Materials for MI Treatment: Could "The Matrix" Really Be the Future?J Am Coll Cardiol. 2016 Mar 8;67(9):1087-1090. doi: 10.1016/j.jacc.2016.01.006. J Am Coll Cardiol. 2016. PMID: 26940930 No abstract available.
References
-
- Delewi R, Andriessen A, Tijssen JGP, Zijlstra F, Piek JJ, Hirsch A. Impact of intracoronary cell therapy on left ventricular function in the setting of acute myocardial infarction: a meta-analysis of randomised controlled clinical trials. Heart. 2013;99:225–232. - PubMed
-
- Rane AA, Christman KL. Biomaterials for the treatment of myocardial infarction a 5-year update. J Am Coll Cardiol. 2011;58:2615–2629. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

