A transdisciplinary model to inform randomized clinical trial methods for electronic cigarette evaluation
- PMID: 26941050
- PMCID: PMC4778292
- DOI: 10.1186/s12889-016-2792-8
A transdisciplinary model to inform randomized clinical trial methods for electronic cigarette evaluation
Abstract
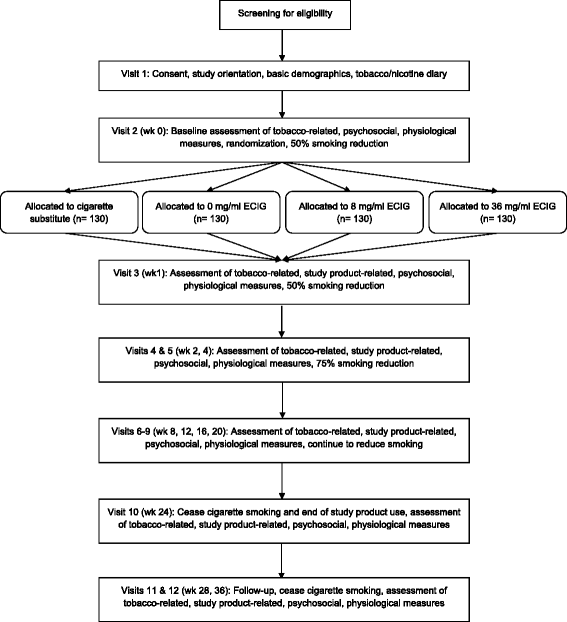
Background: This study is a systematic evaluation of a novel tobacco product, electronic cigarettes (ECIGs) using a two-site, four-arm, 6-month, parallel-group randomized controlled trial (RCT) with a follow-up to 9 months. Virginia Commonwealth University is the primary site and Penn State University is the secondary site. This RCT design is important because it is informed by analytical work, clinical laboratory results, and qualitative/quantitative findings regarding the specific ECIG products used.
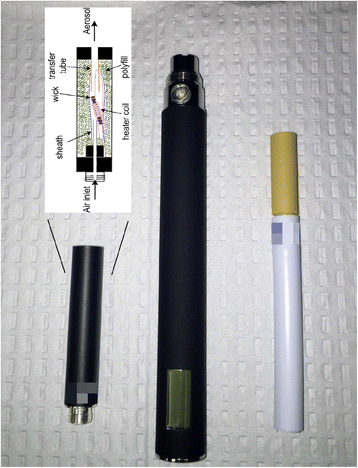
Methods: Participants (N = 520) will be randomized across sites and must be healthy smokers of >9 cigarettes for at least one year, who have not had a quit attempt in the prior month, are not planning to quit in the next 6 months, and are interested in reducing cigarette intake. Participants will be randomized into one of four 24-week conditions: a cigarette substitute that does not produce an inhalable aerosol; or one of three ECIG conditions that differ by nicotine concentration 0, 8, or 36 mg/ml. Blocked randomization will be accomplished with a 1:1:1:1 ratio of condition assignments at each site. Specific aims are to: characterize ECIG influence on toxicants, biomarkers, health indicators, and disease risk; determine tobacco abstinence symptom and adverse event profile associated with real-world ECIG use; and examine the influence of ECIG use on conventional tobacco product use. Liquid nicotine concentration-related differences on these study outcomes are predicted. Participants and research staff in contact with participants will be blinded to the nicotine concentration in the ECIG conditions.
Discussion: Results from this study will inform knowledge concerning ECIG use as well as demonstrate a model that may be applied to other novel tobacco products. The model of using prior empirical testing of ECIG devices should be considered in other RCT evaluations.
Trial registration: TRN: NCT02342795 , registered December 16, 2014.
Figures
References
-
- McRobbie H, Phillips A, Goniewicz ML, Smith KM, Knight-West O, Przulj D, Hajek P. Effects of switching to electronic cigarettes with and without concurrent smoking on exposure to nicotine, carbon monoxide, and acrolein. Cancer Prev Res (Phila) 2015;8:873–8. doi: 10.1158/1940-6207.CAPR-15-0058. - DOI - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

