Chemotherapy with or without autologous cytokine-induced killer cell transfusion as the first-line treatment for stage IV gastrointestinal cancer: a phase II clinical trial
- PMID: 26941189
- PMCID: PMC11818974
- DOI: 10.1007/s00432-016-2127-2
Chemotherapy with or without autologous cytokine-induced killer cell transfusion as the first-line treatment for stage IV gastrointestinal cancer: a phase II clinical trial
Abstract
Objective: To investigate the efficacy of autologous cytokine-induced killer (CIK) cell therapy combined with chemotherapy versus chemotherapy alone for the treatment of stage IV gastrointestinal (GI) cancer in the first-line setting.
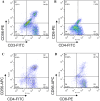
Methods: Thirty-three patients diagnosed with stage IV GI cancer were divided into chemotherapy plus CIK group (chemo-CIK, n = 16) and chemotherapy-alone group (chemo-alone, n = 17). Autologous peripheral blood mononuclear cells were separated by flow cytometry, cultured in vitro to induce CIK cells, and transfused into patients on days 14 and 16 of the first and second chemotherapy cycles.
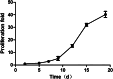
Results: The median progression-free survival (PFS) was 5.6 months for patients in the chemo-CIK group and 3.83 months for those in the chemo-alone group. The difference was borderline significant (P = 0.06), indicating a potential advantage for combined CIK cell transfusion with chemotherapy in improving PFS. A favored objective response rate was also observed in the chemo-CIK group than in the chemo-alone group. This study also revealed that CIK cell transfusion restored the cellular immunity in these GI cancer patients. The percentage of natural killer T cells, NK cells, CD3(+) T cells, and T-cell subgroups CD4(+) proportion in the peripheral blood of cancer patients significantly increased after the CIK cell transfusion, while the change in T-cell subgroups CD8(+) and CD4(+)/CD8(+) did not differ significantly.
Conclusions: The study showed that the addition of CIK cell transfusion to traditional chemotherapy in the first-line setting was associated with a prolonged PFS and enhanced T-lymphocyte subset activity, supporting a potential treatment choice for advanced GI cancer patients.
Keywords: CIK cell; Chemotherapy; Clinical trial; Gastrointestinal cancer.
Conflict of interest statement
None of the authors declare any conflicts of interest in relation to this submission.
Figures


Similar articles
-
Inhibition of human gastric cancer growth by cytokine-induced killer cells plus chemotherapy with or without cadonilimab in a mouse xenograft tumor model.Front Immunol. 2025 Jun 5;16:1609320. doi: 10.3389/fimmu.2025.1609320. eCollection 2025. Front Immunol. 2025. PMID: 40539041 Free PMC article.
-
Cytokine-induced killer cells in the treatment of patients with solid carcinomas: a systematic review and pooled analysis.Cytotherapy. 2012 Apr;14(4):483-93. doi: 10.3109/14653249.2011.649185. Epub 2012 Jan 25. Cytotherapy. 2012. PMID: 22277010
-
Preoperative NLR for predicting survival rate after radical resection combined with adjuvant immunotherapy with CIK and postoperative chemotherapy in gastric cancer.J Cancer Res Clin Oncol. 2017 May;143(5):861-871. doi: 10.1007/s00432-016-2330-1. Epub 2017 Jan 20. J Cancer Res Clin Oncol. 2017. PMID: 28108815 Free PMC article. Clinical Trial.
-
The effectiveness and cost-effectiveness of carmustine implants and temozolomide for the treatment of newly diagnosed high-grade glioma: a systematic review and economic evaluation.Health Technol Assess. 2007 Nov;11(45):iii-iv, ix-221. doi: 10.3310/hta11450. Health Technol Assess. 2007. PMID: 17999840
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
Cited by
-
Clinical Applications of Combined Immunotherapy Approaches in Gastrointestinal Cancer: A Case-Based Review.Vaccines (Basel). 2023 Sep 29;11(10):1545. doi: 10.3390/vaccines11101545. Vaccines (Basel). 2023. PMID: 37896948 Free PMC article. Review.
-
Cytokine-induced killer cells mediated pathways in the treatment of colorectal cancer.Cell Commun Signal. 2022 Mar 28;20(1):41. doi: 10.1186/s12964-022-00836-0. Cell Commun Signal. 2022. PMID: 35346234 Free PMC article. Review.
-
Cytokine-induced killer cells/natural killer cells combined with anti-GD2 monoclonal antibody increase cell death rate in neuroblastoma SK-N-SH cells.Oncol Lett. 2019 Dec;18(6):6525-6535. doi: 10.3892/ol.2019.11020. Epub 2019 Oct 29. Oncol Lett. 2019. PMID: 31807172 Free PMC article.
-
NK and cells with NK-like activities in cancer immunotherapy-clinical perspectives.Med Oncol. 2022 Jun 18;39(9):131. doi: 10.1007/s12032-022-01735-7. Med Oncol. 2022. PMID: 35716327 Review.
References
-
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM (2010) Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 127(12):2893–2917 - PubMed
-
- Hercend T, Griffin JD, Bensussan A, Schmidt RE, Edson MA, Brennan A et al (1985) Generation of monoclonal antibodies to a human natural killer clone. Characterization of two natural killer associated antigens, NKH1A and NKH2, expressed on subsets of large granular lymphocytes. J Clin Invest 75:932–943 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

