Sialic acid-modified antigens impose tolerance via inhibition of T-cell proliferation and de novo induction of regulatory T cells
- PMID: 26941238
- PMCID: PMC4812702
- DOI: 10.1073/pnas.1507706113
Sialic acid-modified antigens impose tolerance via inhibition of T-cell proliferation and de novo induction of regulatory T cells
Abstract
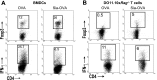
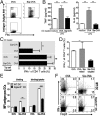
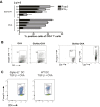
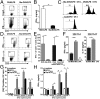
Sialic acids are negatively charged nine-carbon carboxylated monosaccharides that often cap glycans on glycosylated proteins and lipids. Because of their strategic location at the cell surface, sialic acids contribute to interactions that are critical for immune homeostasis via interactions with sialic acid-binding Ig-type lectins (siglecs). In particular, these interactions may be of importance in cases where sialic acids may be overexpressed, such as on certain pathogens and tumors. We now demonstrate that modification of antigens with sialic acids (Sia-antigens) regulates the generation of antigen-specific regulatory T (Treg) cells via dendritic cells (DCs). Additionally, DCs that take up Sia-antigen prevent formation of effector CD4(+) and CD8(+)T cells. Importantly, the regulatory properties endowed on DCs upon Sia-antigen uptake are antigen-specific: only T cells responsive to the sialylated antigen become tolerized. In vivo, injection of Sia-antigen-loaded DCs increased de novo Treg-cell numbers and dampened effector T-cell expansion and IFN-γ production. The dual tolerogenic features that Sia-antigen imposed on DCs are Siglec-E-mediated and maintained under inflammatory conditions. Moreover, loading DCs with Sia-antigens not only inhibited the function of in vitro-established Th1 and Th17 effector T cells but also significantly dampened ex vivo myelin-reactive T cells, present in the circulation of mice with experimental autoimmune encephalomyelitis. These data indicate that sialic acid-modified antigens instruct DCs in an antigen-specific tolerogenic programming, enhancing Treg cells and reducing the generation and propagation of inflammatory T cells. Our data suggest that sialylation of antigens provides an attractive way to induce antigen-specific immune tolerance.
Keywords: dendritic cells; glycans; regulatory T cells; sialic acids; tolerance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155(3):1151–1164. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

