Subcellular optogenetic activation of Cdc42 controls local and distal signaling to drive immune cell migration
- PMID: 26941336
- PMCID: PMC4850032
- DOI: 10.1091/mbc.E15-12-0832
Subcellular optogenetic activation of Cdc42 controls local and distal signaling to drive immune cell migration
Abstract
Migratory immune cells use intracellular signaling networks to generate and orient spatially polarized responses to extracellular cues. The monomeric G protein Cdc42 is believed to play an important role in controlling the polarized responses, but it has been difficult to determine directly the consequences of localized Cdc42 activation within an immune cell. Here we used subcellular optogenetics to determine how Cdc42 activation at one side of a cell affects both cell behavior and dynamic molecular responses throughout the cell. We found that localized Cdc42 activation is sufficient to generate polarized signaling and directional cell migration. The optically activated region becomes the leading edge of the cell, with Cdc42 activating Rac and generating membrane protrusions driven by the actin cytoskeleton. Cdc42 also exerts long-range effects that cause myosin accumulation at the opposite side of the cell and actomyosin-mediated retraction of the cell rear. This process requires the RhoA-activated kinase ROCK, suggesting that Cdc42 activation at one side of a cell triggers increased RhoA signaling at the opposite side. Our results demonstrate how dynamic, subcellular perturbation of an individual signaling protein can help to determine its role in controlling polarized cellular responses.
© 2016 O’Neill et al. This article is distributed by The American Society for Cell Biology under license from the author(s). Two months after publication it is available to the public under an Attribution–Noncommercial–Share Alike 3.0 Unported Creative Commons License (http://creativecommons.org/licenses/by-nc-sa/3.0).
Figures


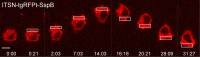






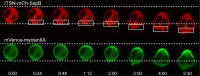

Similar articles
-
PLEKHG3 enhances polarized cell migration by activating actin filaments at the cell front.Proc Natl Acad Sci U S A. 2016 Sep 6;113(36):10091-6. doi: 10.1073/pnas.1604720113. Epub 2016 Aug 23. Proc Natl Acad Sci U S A. 2016. PMID: 27555588 Free PMC article.
-
Shear stress-induced endothelial cell polarization is mediated by Rho and Rac but not Cdc42 or PI 3-kinases.J Cell Biol. 2003 Apr 28;161(2):429-39. doi: 10.1083/jcb.200210135. J Cell Biol. 2003. PMID: 12719476 Free PMC article.
-
Cdc42 controls the polarity of the actin and microtubule cytoskeletons through two distinct signal transduction pathways.J Cell Sci. 2005 Jun 15;118(Pt 12):2579-87. doi: 10.1242/jcs.02385. Epub 2005 May 31. J Cell Sci. 2005. PMID: 15928049
-
Dynamic functions of RhoA in tumor cell migration and invasion.Small GTPases. 2013 Jul-Sep;4(3):141-7. doi: 10.4161/sgtp.25131. Epub 2013 Jun 10. Small GTPases. 2013. PMID: 24025634 Free PMC article. Review.
-
FilGAP and its close relatives: a mediator of Rho-Rac antagonism that regulates cell morphology and migration.Biochem J. 2013 Jul 1;453(1):17-25. doi: 10.1042/BJ20130290. Biochem J. 2013. PMID: 23763313 Review.
Cited by
-
In-silico predicted mouse melanopsins with blue spectral shifts deliver efficient subcellular signaling.Cell Commun Signal. 2024 Aug 8;22(1):394. doi: 10.1186/s12964-024-01753-0. Cell Commun Signal. 2024. PMID: 39118111 Free PMC article.
-
The spatial distribution of GPCR and Gβγ activity across a cell dictates PIP3 dynamics.Sci Rep. 2023 Feb 16;13(1):2771. doi: 10.1038/s41598-023-29639-0. Sci Rep. 2023. PMID: 36797332 Free PMC article.
-
Excitable Signal Transduction Networks in Directed Cell Migration.Annu Rev Cell Dev Biol. 2017 Oct 6;33:103-125. doi: 10.1146/annurev-cellbio-100616-060739. Epub 2017 Aug 9. Annu Rev Cell Dev Biol. 2017. PMID: 28793794 Free PMC article. Review.
-
Spatiotemporal dynamics of membrane surface charge regulates cell polarity and migration.Nat Cell Biol. 2022 Oct;24(10):1499-1515. doi: 10.1038/s41556-022-00997-7. Epub 2022 Oct 6. Nat Cell Biol. 2022. PMID: 36202973 Free PMC article.
-
On the influence of cell shape on dynamic reaction-diffusion polarization patterns.PLoS One. 2021 Mar 18;16(3):e0248293. doi: 10.1371/journal.pone.0248293. eCollection 2021. PLoS One. 2021. PMID: 33735291 Free PMC article.
References
-
- Arber S, Barbayannis FA, Hanser H, Schneider C, Stanyon CA, Bernard O, Caroni P. Regulation of actin dynamics through phosphorylation of cofilin by LIM-kinase. Nature. 1998;393:805–809. - PubMed
-
- Benard V, Bohl BP, Bokoch GM. Characterization of rac and cdc42 activation in chemoattractant-stimulated human neutrophils using a novel assay for active GTPases. J Biol Chem. 1999;274:13198–13204. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

