Early Development of Functional Network Segregation Revealed by Connectomic Analysis of the Preterm Human Brain
- PMID: 26941380
- PMCID: PMC6059235
- DOI: 10.1093/cercor/bhw038
Early Development of Functional Network Segregation Revealed by Connectomic Analysis of the Preterm Human Brain
Abstract
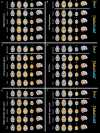
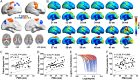
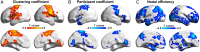
Human brain functional networks are topologically organized with nontrivial connectivity characteristics such as small-worldness and densely linked hubs to support highly segregated and integrated information processing. However, how they emerge and change at very early developmental phases remains poorly understood. Here, we used resting-state functional MRI and voxel-based graph theory analysis to systematically investigate the topological organization of whole-brain networks in 40 infants aged around 31 to 42 postmenstrual weeks. The functional connectivity strength and heterogeneity increased significantly in primary motor, somatosensory, visual, and auditory regions, but much less in high-order default-mode and executive-control regions. The hub and rich-club structures in primary regions were already present at around 31 postmenstrual weeks and exhibited remarkable expansions with age, accompanied by increased local clustering and shortest path length, indicating a transition from a relatively random to a more organized configuration. Moreover, multivariate pattern analysis using support vector regression revealed that individual brain maturity of preterm babies could be predicted by the network connectivity patterns. Collectively, we highlighted a gradually enhanced functional network segregation manner in the third trimester, which is primarily driven by the rapid increases of functional connectivity of the primary regions, providing crucial insights into the topological development patterns prior to birth.
Keywords: connectome; functional connectivity; hub; preterm; rich club.
© The Author 2016. Published by Oxford University Press. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures






References
-
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. 1995. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 34:537–541. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

