Diverse Synaptic Distributions of G Protein-coupled Estrogen Receptor 1 in Monkey Prefrontal Cortex with Aging and Menopause
- PMID: 26941383
- PMCID: PMC5909633
- DOI: 10.1093/cercor/bhw050
Diverse Synaptic Distributions of G Protein-coupled Estrogen Receptor 1 in Monkey Prefrontal Cortex with Aging and Menopause
Abstract
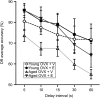
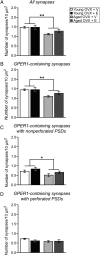
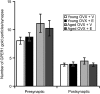
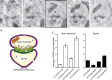
Age- and menopause-related impairment in working memory mediated by the dorsolateral prefrontal cortex (dlPFC) occurs in humans and nonhuman primates. Long-term cyclic 17β-estradiol treatment rescues cognitive deficits in aged ovariectomized rhesus monkeys while restoring highly plastic synapses. Here we tested whether distributions of G protein-coupled estrogen receptor 1 (GPER1) within monkey layer III dlPFC synapses are sensitive to age and estradiol, and coupled to cognitive function. Ovariectomized young and aged monkeys administered vehicle or estradiol were first tested on a delayed response test of working memory. Then, quantitative serial section immunoelectron microscopy was used to determine the distributions of synaptic GPER1. GPER1-containing nonperforated axospinous synapse density was reduced with age, and partially restored with estrogen treatment. The majority of synapses expressed GPER1, which was predominately localized to presynaptic cytoplasm and mitochondria. GPER1 was also abundant at plasmalemmas, and within cytoplasmic and postsynaptic density (PSD) domains of dendritic spines. GPER1 levels did not differ with age or treatment, and none of the variables examined were tightly associated with cognitive function. However, greater representation of GPER1 subjacent to the PSD accompanied higher synapse density. These data suggest that GPER1 is positioned to support diverse functions key to synaptic plasticity in monkey dlPFC.
Keywords: Area 46; GPER1; delayed response; estradiol; synapse.
© The Author 2016. Published by Oxford University Press. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures

Similar articles
-
Synaptic distributions of pS214-tau in rhesus monkey prefrontal cortex are associated with spine density, but not with cognitive decline.J Comp Neurol. 2019 Mar 1;527(4):856-873. doi: 10.1002/cne.24576. Epub 2018 Dec 4. J Comp Neurol. 2019. PMID: 30408169 Free PMC article.
-
Estrogen Alters the Synaptic Distribution of Phospho-GluN2B in the Dorsolateral Prefrontal Cortex While Promoting Working Memory in Aged Rhesus Monkeys.Neuroscience. 2018 Dec 1;394:303-315. doi: 10.1016/j.neuroscience.2018.09.021. Neuroscience. 2018. PMID: 30482274 Free PMC article.
-
Estrogen Restores Multisynaptic Boutons in the Dorsolateral Prefrontal Cortex while Promoting Working Memory in Aged Rhesus Monkeys.J Neurosci. 2016 Jan 20;36(3):901-10. doi: 10.1523/JNEUROSCI.3480-13.2016. J Neurosci. 2016. PMID: 26791219 Free PMC article.
-
Interactive effects of age and estrogen on cortical neurons: implications for cognitive aging.Neuroscience. 2011 Sep 15;191:148-58. doi: 10.1016/j.neuroscience.2011.05.045. Epub 2011 Jun 2. Neuroscience. 2011. PMID: 21664255 Free PMC article. Review.
-
Estrogen Effects on Cognitive and Synaptic Health Over the Lifecourse.Physiol Rev. 2015 Jul;95(3):785-807. doi: 10.1152/physrev.00036.2014. Physiol Rev. 2015. PMID: 26109339 Free PMC article. Review.
Cited by
-
Neuronal properties of pyramidal cells in lateral prefrontal cortex of the aging rhesus monkey brain are associated with performance deficits on spatial working memory but not executive function.Geroscience. 2023 Jun;45(3):1317-1342. doi: 10.1007/s11357-023-00798-2. Epub 2023 Apr 28. Geroscience. 2023. PMID: 37106282 Free PMC article.
-
Synaptic distributions of pS214-tau in rhesus monkey prefrontal cortex are associated with spine density, but not with cognitive decline.J Comp Neurol. 2019 Mar 1;527(4):856-873. doi: 10.1002/cne.24576. Epub 2018 Dec 4. J Comp Neurol. 2019. PMID: 30408169 Free PMC article.
-
On the role of sex steroids in biological functions by classical and non-classical pathways. An update.Front Neuroendocrinol. 2021 Jul;62:100926. doi: 10.1016/j.yfrne.2021.100926. Epub 2021 Jun 3. Front Neuroendocrinol. 2021. PMID: 34089761 Free PMC article. Review.
-
Attenuation of estrogen and its receptors in the post-menopausal stage exacerbates dyslipidemia and leads to cognitive impairment.Mol Brain. 2023 Nov 20;16(1):80. doi: 10.1186/s13041-023-01068-0. Mol Brain. 2023. PMID: 37986006 Free PMC article.
-
Estrogen Alters the Synaptic Distribution of Phospho-GluN2B in the Dorsolateral Prefrontal Cortex While Promoting Working Memory in Aged Rhesus Monkeys.Neuroscience. 2018 Dec 1;394:303-315. doi: 10.1016/j.neuroscience.2018.09.021. Neuroscience. 2018. PMID: 30482274 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

