Rheotaxis of Larval Zebrafish: Behavioral Study of a Multi-Sensory Process
- PMID: 26941620
- PMCID: PMC4763089
- DOI: 10.3389/fnsys.2016.00014
Rheotaxis of Larval Zebrafish: Behavioral Study of a Multi-Sensory Process
Abstract
Awake animals unceasingly perceive sensory inputs with great variability of nature and intensity, and understanding how the nervous system manages this continuous flow of diverse information to get a coherent representation of the environment is arguably a central question in systems neuroscience. Rheotaxis, the ability shared by most aquatic species to orient toward a current and swim to hold position, is an innate and robust multi-sensory behavior that is known to involve the lateral line and visual systems. To facilitate the neuroethological study of rheotaxic behavior in larval zebrafish we developed an assay for freely swimming larvae that allows for high experimental throughtput, large statistic and a fine description of the behavior. We show that there exist a clear transition from exploration to counterflow swim, and by changing the sensory modalities accessible to the fishes (visual only, lateral line only or both) and comparing the swim patterns at different ages we were able to detect and characterize two different mechanisms for position holding, one mediated by the lateral line and one mediated by the visual system. We also found that when both sensory modalities are accessible the visual system overshadows the lateral line, suggesting that at the larval stage the sensory inputs are not merged to finely tune the behavior but that redundant information pathways may be used as functional fallbacks.
Keywords: behavior; lateral line; multi-sensory integration; rheotaxis; vision; zebrafish.
Figures
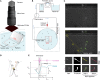
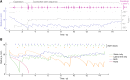

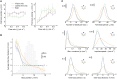
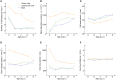
References
-
- Arnold G. Weihs, D. (1977). The hydrodynamics of rheotaxis in the plaice (Pleuronectes Platessa L.). J. Exp. Biol. 75, 147–169.
-
- Arnold G. P. (1974). Rhoetropism in fishes. Biol. Rev. 49, 515–576. - PubMed
-
- Baker C. Montgomery, J. (1999). The sensory basis of rheotaxis in the blind Mexican cave fish, Astyanax fasciatus. J. Comp. Physiol. A 184, 519–527.
LinkOut - more resources
Full Text Sources
Other Literature Sources

