Feces Derived Allergens of Tyrophagus putrescentiae Reared on Dried Dog Food and Evidence of the Strong Nutritional Interaction between the Mite and Bacillus cereus Producing Protease Bacillolysins and Exo-chitinases
- PMID: 26941650
- PMCID: PMC4764834
- DOI: 10.3389/fphys.2016.00053
Feces Derived Allergens of Tyrophagus putrescentiae Reared on Dried Dog Food and Evidence of the Strong Nutritional Interaction between the Mite and Bacillus cereus Producing Protease Bacillolysins and Exo-chitinases
Abstract
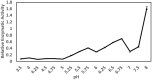
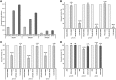
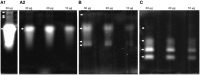
Tyrophagus putrescentiae (Schrank, 1781) is an emerging source of allergens in stored products and homes. Feces proteases are the major allergens of astigmatid mites (Acari: Acaridida). In addition, the mites are carriers of microorganisms and microbial adjuvant compounds that stimulate innate signaling pathways. We sought to analyze the mite feces proteome, proteolytic activities, and mite-bacterial interaction in dry dog food (DDF). Proteomic methods comprising enzymatic and zymographic analysis of proteases and 2D-E-MS/MS were performed. The highest protease activity was assigned to trypsin-like proteases; lower activity was assigned to chymotrypsin-like proteases, and the cysteine protease cathepsin B-like had very low activity. The 2D-E-MS/MS proteomic analysis identified mite trypsin allergen Tyr p3, fatty acid-binding protein Tyr p13 and putative mite allergens ferritin (Grp 30) and (poly)ubiquitins. Tyr p3 was detected at different positions of the 2D-E. It indicates presence of zymogen at basic pI, and mature-enzyme form and enzyme fragment at acidic pI. Bacillolysins (neutral and alkaline proteases) of Bacillus cereus symbiont can contribute to the protease activity of the mite extract. The bacterial exo-chitinases likely contribute to degradation of mite exuviae, mite bodies or food boluses consisting of chitin, including the peritrophic membrane. Thus, the chitinases disrupt the feces and facilitate release of the allergens. B. cereus was isolated and identified based on amplification and sequencing of 16S rRNA and motB genes. B. cereus was added into high-fat, high-protein (DDF) and low-fat, low-protein (flour) diets to 1 and 5% (w/w), and the diets palatability was evaluated in 21-day population growth test. The supplementation of diet with B. cereus significantly suppressed population growth and the suppressive effect was higher in the high-fat, high-protein diet than in the low-fat, low-protein food. Thus, B. cereus has to coexist with the mite in balance to be beneficial for the mite. The mite-B. cereus symbiosis can be beneficial-suppressive at some level. The results increase the veterinary and medical importance of the allergens detected in feces. The B. cereus enzymes/toxins are important components of mite allergens. The strong symbiotic association of T. putrescentiae with B. cereus in DDF was indicated.
Keywords: Bacillus cereus; Tyrophagus putrescentiae; allergen; bacillolysin; exochitinase; nutrition; protease; symbiosis.
Figures
Similar articles
-
Label-free proteomic analysis reveals differentially expressed Wolbachia proteins in Tyrophagus putrescentiae: Mite allergens and markers reflecting population-related proteome differences.J Proteomics. 2021 Oct 30;249:104356. doi: 10.1016/j.jprot.2021.104356. Epub 2021 Aug 23. J Proteomics. 2021. PMID: 34438106
-
Detailed two-dimensional gel proteomic mapping of the feces of the house dust mite Dermatophagoides pteronyssinus and comparison with D. farinae: Reduced trypsin protease content in D. pteronyssinus and different isoforms.J Proteomics. 2017 Jun 6;162:11-19. doi: 10.1016/j.jprot.2017.04.021. Epub 2017 Apr 23. J Proteomics. 2017. PMID: 28442447
-
Population Growth of the Generalist Mite Tyrophagus putrescentiae (Acari: Acaridida) Following Adaptation to High- or Low-Fat and High- or Low-Protein Diets and the Effect of Dietary Switch.Environ Entomol. 2015 Dec;44(6):1599-604. doi: 10.1093/ee/nvv129. Epub 2015 Aug 7. Environ Entomol. 2015. PMID: 26314031
-
Critically Appraised Topic on Adverse Food Reactions of Companion Animals (8): Storage Mites in Commercial Pet foods.BMC Vet Res. 2019 Oct 31;15(1):385. doi: 10.1186/s12917-019-2102-7. BMC Vet Res. 2019. PMID: 31672139 Free PMC article. Review.
-
The Role of Dust Mites in Allergy.Clin Rev Allergy Immunol. 2019 Dec;57(3):312-329. doi: 10.1007/s12016-018-8693-0. Clin Rev Allergy Immunol. 2019. PMID: 29936683 Review.
Cited by
-
The Microbiota of a Mite Prey-Predator System on Different Host Plants Are Characterized by Dysbiosis and Potential Functional Redundancy.Microb Ecol. 2023 May;85(4):1590-1607. doi: 10.1007/s00248-022-02032-6. Epub 2022 May 11. Microb Ecol. 2023. PMID: 35543735
-
The Mite Tyrophagus putrescentiae Hosts Population-Specific Microbiomes That Respond Weakly to Starvation.Microb Ecol. 2019 Feb;77(2):488-501. doi: 10.1007/s00248-018-1224-y. Epub 2018 Jul 2. Microb Ecol. 2019. PMID: 29967922
-
First identification of Tyrophagus curvipenis (Acari: Acaridae) and pathogen detection in Apis mellifera colonies in the Republic of Korea.Sci Rep. 2023 Jun 10;13(1):9469. doi: 10.1038/s41598-023-36695-z. Sci Rep. 2023. PMID: 37301922 Free PMC article.
-
Mixta mediterraneensis as a novel and abundant gut symbiont of the allergen-producing domestic mite Blomia tropicalis.Exp Appl Acarol. 2024 Feb;92(2):161-181. doi: 10.1007/s10493-023-00875-3. Epub 2024 Jan 16. Exp Appl Acarol. 2024. PMID: 38227156 Free PMC article.
-
Effects of Local Nasal Immunotherapy with FIP-fve Peptide and Denatured Tyrophagus putrescentiae for Storage Mite-Induced Airway Inflammation.Arch Immunol Ther Exp (Warsz). 2022 Jan 31;70(1):6. doi: 10.1007/s00005-022-00645-w. Arch Immunol Ther Exp (Warsz). 2022. PMID: 35099617
References
-
- Adigüzel A. C., Bitlisli B. O., Yasa I., Eriksen N. T. (2009). Sequential secretion of collagenolytic, elastolytic, and keratinolytic proteases in peptide-limited cultures of two Bacillus cereus strains isolated from wool. J. Appl. Microbiol. 107, 226–234. 10.1111/j.1365-2672.2009.04200.x - DOI - PubMed
-
- Altincicek B., Linder M., Linder D., Preissner K. T., Vilcinskas A. (2007). Microbial metalloproteinases mediate sensing of invading pathogens and activate innate immune responses in the lepidopteran model host Galleria mellonella. Infect. Immun. 75, 175–183. 10.1128/IAI.01385-06 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

