Hydrogen Production and Enzyme Activities in the Hyperthermophile Thermococcus paralvinellae Grown on Maltose, Tryptone, and Agricultural Waste
- PMID: 26941713
- PMCID: PMC4762990
- DOI: 10.3389/fmicb.2016.00167
Hydrogen Production and Enzyme Activities in the Hyperthermophile Thermococcus paralvinellae Grown on Maltose, Tryptone, and Agricultural Waste
Abstract
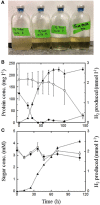
Thermococcus may be an important alternative source of H2 in the hot subseafloor in otherwise low H2 environments such as some hydrothermal vents and oil reservoirs. It may also be useful in industry for rapid agricultural waste treatment and concomitant H2 production. Thermococcus paralvinellae grown at 82°C without sulfur produced up to 5 mmol of H2 L(-1) at rates of 5-36 fmol H2 cell(-1) h(-1) on 0.5% (wt vol(-1)) maltose, 0.5% (wt vol(-1)) tryptone, and 0.5% maltose + 0.05% tryptone media. Two potentially inhibiting conditions, the presence of 10 mM acetate and low pH (pH 5) in maltose-only medium, did not significantly affect growth or H2 production. Growth rates, H2 production rates, and cell yields based on H2 production were the same as those for Pyrococcus furiosus grown at 95°C on the same media for comparison. Acetate, butyrate, succinate, isovalerate, and formate were also detected as end products. After 100 h, T. paralvinellae produced up to 5 mmol of H2 L(-1) of medium when grown on up to 70% (vol vol(-1)) waste milk from cows undergoing treatment for mastitis with the bacterial antibiotic Ceftiofur and from untreated cows. The amount of H2 produced by T. paralvinellae increased with increasing waste concentrations, but decreased in P. furiosus cultures supplemented with waste milk above 1% concentration. All mesophilic bacteria from the waste milk that grew on Luria Bertani, Sheep's Blood (selective for Staphylococcus, the typical cause of mastitis), and MacConkey (selective for Gram-negative enteric bacteria) agar plates were killed by heat during incubation at 82°C. Ceftiofur, which is heat labile, was below the detection limit following incubation at 82°C. T. paralvinellae also produced up to 6 mmol of H2 L(-1) of medium when grown on 0.1-10% (wt vol(-1)) spent brewery grain while P. furiosus produced < 1 mmol of H2 L(-1). Twelve of 13 enzyme activities in T. paralvinellae showed significant (p < 0.05) differences across six different growth conditions; however, methyl viologen-dependent membrane hydrogenase activity remained constant across all media types. The results demonstrate the potential of at least some Thermococcus species to produce H2 if protein and α-glucosides are present as substrates.
Keywords: Thermococcus; bioenergy; hydrogenase; hyperthermophile; waste remediation.
Figures

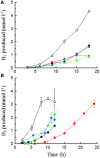
 ), 0.5% maltose + 0.05% tryptone (●), 0.5% maltose + 10 mM acetate (
), 0.5% maltose + 0.05% tryptone (●), 0.5% maltose + 10 mM acetate ( ), and 0.5% maltose at pH 5.0 (
), and 0.5% maltose at pH 5.0 ( ). Error bars represent the standard error.
). Error bars represent the standard error.


 ), 0.5% maltose + 0.05% tryptone (○), 0.5% maltose + 10 mM acetate (
), 0.5% maltose + 0.05% tryptone (○), 0.5% maltose + 10 mM acetate ( ), 0.5% maltose at pH 5.0 (
), 0.5% maltose at pH 5.0 ( ), and 0.1% waste milk (
), and 0.1% waste milk ( ). Data are examples from individual bioreactor reactions.
). Data are examples from individual bioreactor reactions.Similar articles
-
Growth Kinetics, Carbon Isotope Fractionation, and Gene Expression in the Hyperthermophile Methanocaldococcus jannaschii during Hydrogen-Limited Growth and Interspecies Hydrogen Transfer.Appl Environ Microbiol. 2019 Apr 18;85(9):e00180-19. doi: 10.1128/AEM.00180-19. Print 2019 May 1. Appl Environ Microbiol. 2019. PMID: 30824444 Free PMC article.
-
Formate addition enhanced hydrogen production by Thermococcus paralvinellae when grown on brewery wastewater.Front Microbiol. 2025 Mar 18;16:1560780. doi: 10.3389/fmicb.2025.1560780. eCollection 2025. Front Microbiol. 2025. PMID: 40170917 Free PMC article.
-
Formate hydrogenlyase and formate secretion ameliorate H2 inhibition in the hyperthermophilic archaeon Thermococcus paralvinellae.Environ Microbiol. 2018 Mar;20(3):949-957. doi: 10.1111/1462-2920.14022. Epub 2017 Dec 29. Environ Microbiol. 2018. PMID: 29235714
-
Hydrogen Limitation and Syntrophic Growth among Natural Assemblages of Thermophilic Methanogens at Deep-sea Hydrothermal Vents.Front Microbiol. 2016 Aug 5;7:1240. doi: 10.3389/fmicb.2016.01240. eCollection 2016. Front Microbiol. 2016. PMID: 27547206 Free PMC article.
-
Upflow anaerobic sludge blanket reactor--a review.Indian J Environ Health. 2001 Apr;43(2):1-82. Indian J Environ Health. 2001. PMID: 12397675 Review.
Cited by
-
Formate and hydrogen in hydrothermal vents and their use by extremely thermophilic methanogens and heterotrophs.Front Microbiol. 2023 Mar 6;14:1093018. doi: 10.3389/fmicb.2023.1093018. eCollection 2023. Front Microbiol. 2023. PMID: 36950162 Free PMC article. Review.
-
Development of an Effective 6-Methylpurine Counterselection Marker for Genetic Manipulation in Thermococcus barophilus.Genes (Basel). 2018 Feb 7;9(2):77. doi: 10.3390/genes9020077. Genes (Basel). 2018. PMID: 29414865 Free PMC article.
-
Biotechnology of extremely thermophilic archaea.FEMS Microbiol Rev. 2018 Sep 1;42(5):543-578. doi: 10.1093/femsre/fuy012. FEMS Microbiol Rev. 2018. PMID: 29945179 Free PMC article. Review.
-
Growth Kinetics, Carbon Isotope Fractionation, and Gene Expression in the Hyperthermophile Methanocaldococcus jannaschii during Hydrogen-Limited Growth and Interspecies Hydrogen Transfer.Appl Environ Microbiol. 2019 Apr 18;85(9):e00180-19. doi: 10.1128/AEM.00180-19. Print 2019 May 1. Appl Environ Microbiol. 2019. PMID: 30824444 Free PMC article.
-
Solid-State Fermentation: Applications and Future Perspectives for Biostimulant and Biopesticides Production.Microorganisms. 2023 May 26;11(6):1408. doi: 10.3390/microorganisms11061408. Microorganisms. 2023. PMID: 37374910 Free PMC article. Review.
References
-
- Adams M. W. W., Holden J. F., Menon A. L., Schut G. J., Grunden A. M., Hou C., et al. . (2001). Key role for sulfur in peptide metabolism and in regulation of three hydrogenases in the hyperthermophilic archaeon Pyrococcus furiosus. J. Bacteriol. 183, 716–724. 10.1128/JB.183.2.716-724.2001 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

