FHY3 and FAR1 Act Downstream of Light Stable Phytochromes
- PMID: 26941752
- PMCID: PMC4761848
- DOI: 10.3389/fpls.2016.00175
FHY3 and FAR1 Act Downstream of Light Stable Phytochromes
Abstract
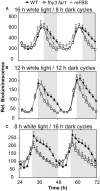
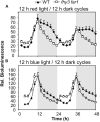
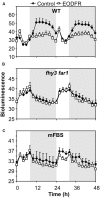
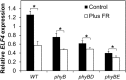
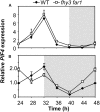
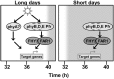
FHY3 and FAR1 are positively acting transcription factors that directly regulate expression of a number of target genes in Arabidopsis thaliana. Here, we looked at the regulation of one specific target gene, ELF4. We demonstrate that the action of FHY3 and FAR1 in upregulation of ELF4 is light dependent. Furthermore, although FHY3 and FAR1 have been exclusively characterized as components of the phytochrome A signaling pathway because of their importance in regulating expression of phyA nuclear importers, we show that, as transcription factors in their own right, FHY3 and FAR1 act downstream of light stable phytochromes, phyB, phyD, and phyE. We demonstrate that light stable phytochrome acts in a red/far-red reversible manner to regulate the level of FHY3 protein. We also observed that ELF4 shows specific FHY3 and FAR1-mediated light induction in the evening and we show that regulation by light stable phytochromes at this time is important as it allows the plant to maintain normal ELF4 expression beyond dusk when the day length shortens, something which would not be possible through light labile phytochrome action. Without FHY3 and FAR1, ELF4 expression falls rapidly at dusk and in short days this results in an early drop in ELF4 expression, accompanied by a de-repression of an ELF4 target gene later in the night. Our results, therefore, demonstrate an important role for FHY3 and FAR1 as mediators of light stable phytochrome signaling.
Keywords: Arabidopsis; light; phytochrome; signal transduction; transcription.
Figures


Similar articles
-
Arabidopsis FHY3 defines a key phytochrome A signaling component directly interacting with its homologous partner FAR1.EMBO J. 2002 Mar 15;21(6):1339-49. doi: 10.1093/emboj/21.6.1339. EMBO J. 2002. PMID: 11889039 Free PMC article.
-
The FHY3 and FAR1 genes encode transposase-related proteins involved in regulation of gene expression by the phytochrome A-signaling pathway.Plant J. 2003 May;34(4):453-71. doi: 10.1046/j.1365-313x.2003.01741.x. Plant J. 2003. PMID: 12753585
-
Arabidopsis thaliana FAR-RED ELONGATED HYPOCOTYLS3 (FHY3) and FAR-RED-IMPAIRED RESPONSE1 (FAR1) modulate starch synthesis in response to light and sugar.New Phytol. 2017 Mar;213(4):1682-1696. doi: 10.1111/nph.14300. Epub 2016 Nov 15. New Phytol. 2017. PMID: 27859295
-
Emerging Roles of FHY3 and FAR1 as System Integrators in Plant Development.Plant Cell Physiol. 2023 Oct 16;64(10):1139-1145. doi: 10.1093/pcp/pcad068. Plant Cell Physiol. 2023. PMID: 37384577 Review.
-
Multifaceted roles of FHY3 and FAR1 in light signaling and beyond.Trends Plant Sci. 2015 Jul;20(7):453-61. doi: 10.1016/j.tplants.2015.04.003. Epub 2015 May 5. Trends Plant Sci. 2015. PMID: 25956482 Review.
Cited by
-
Salicylic Acid-Mediated Disturbance Increases Bacterial Diversity in the Phyllosphere but Is Overcome by a Dominant Core Community.Front Microbiol. 2022 Feb 24;13:809940. doi: 10.3389/fmicb.2022.809940. eCollection 2022. Front Microbiol. 2022. PMID: 35283825 Free PMC article.
-
Thermo-Sensitive Spikelet Defects 1 acclimatizes rice spikelet initiation and development to high temperature.Plant Physiol. 2023 Mar 17;191(3):1684-1701. doi: 10.1093/plphys/kiac576. Plant Physiol. 2023. PMID: 36517254 Free PMC article.
-
Cellular localization of Arabidopsis EARLY FLOWERING3 is responsive to light quality.Plant Physiol. 2022 Sep 28;190(2):1024-1036. doi: 10.1093/plphys/kiac072. Plant Physiol. 2022. PMID: 35191492 Free PMC article.
-
Complex Signaling Networks Underlying Blue-Light-Mediated Floral Transition in Plants.Plants (Basel). 2025 May 20;14(10):1533. doi: 10.3390/plants14101533. Plants (Basel). 2025. PMID: 40431098 Free PMC article. Review.
-
A mobile ELF4 delivers circadian temperature information from shoots to roots.Nat Plants. 2020 Apr;6(4):416-426. doi: 10.1038/s41477-020-0634-2. Epub 2020 Apr 13. Nat Plants. 2020. PMID: 32284549 Free PMC article.
References
-
- Bauer D., Viczian A., Kircher S., Nobis T., Nitschke R., Kunkel T., et al. (2004). Constitutive photomorphogenesis 1 and multiple photoreceptors control degradation of phytochrome interacting factor 3, a transcription factor required for light signaling in Arabidopsis. Plant Cell 16 1433–1445. 10.1105/tpc.021568 - DOI - PMC - PubMed
-
- Casal J. J., Sánchez R. A., Botto J. F. (1998). Modes of action of phytochromes. J. Exp. Bot. 49 127–138. 10.1093/jxb/49.319.127 - DOI
-
- Devlin P. F., Halliday K. J., Whitelam G. C. (1995). “The phytochrome family and their role in the regulation of seed germination,” in Basic and Applied Aspects of Seed Biology, eds Ellis R. H., Black M., Murdoch A. J., Hong T. D. (Dordrecht: Kluwer Academic Publishers; ), 159–171.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

