Overexpression of Soluble Recombinant Human Lysyl Oxidase by Using Solubility Tags: Effects on Activity and Solubility
- PMID: 26942005
- PMCID: PMC4753049
- DOI: 10.1155/2016/5098985
Overexpression of Soluble Recombinant Human Lysyl Oxidase by Using Solubility Tags: Effects on Activity and Solubility
Abstract
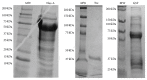
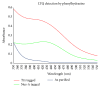
Lysyl oxidase is an important extracellular matrix enzyme that has not been fully characterized due to its low solubility. In order to circumvent the low solubility of this enzyme, three solubility tags (Nus-A, Thioredoxin (Trx), and Glutathione-S-Transferase (GST)) were engineered on the N-terminus of mature lysyl oxidase. Total enzyme yields were determined to be 1.5 mg for the Nus-A tagged enzyme (0.75 mg/L of media), 7.84 mg for the Trx tagged enzyme (3.92 mg/L of media), and 9.33 mg for the GST tagged enzyme (4.67 mg/L of media). Enzymatic activity was calculated to be 0.11 U/mg for the Nus-A tagged enzyme and 0.032 U/mg for the Trx tagged enzyme, and no enzymatic activity was detected for the GST tagged enzyme. All three solubility-tagged forms of the enzyme incorporated copper; however, the GST tagged enzyme appears to bind adventitious copper with greater affinity than the other two forms. The catalytic cofactor, lysyl tyrosyl quinone (LTQ), was determined to be 92% for the Nus-A and Trx tagged lysyl oxidase using the previously reported extinction coefficient of 15.4 mM(-1 )cm(-1). No LTQ was detected for the GST tagged lysyl oxidase. Given these data, it appears that Nus-A is the most suitable tag for obtaining soluble and active recombinant lysyl oxidase from E. coli culture.
Figures

Similar articles
-
Identification of Histidine 303 as the Catalytic Base of Lysyl Oxidase via Site-Directed Mutagenesis.Protein J. 2018 Feb;37(1):47-57. doi: 10.1007/s10930-017-9749-3. Protein J. 2018. PMID: 29127553
-
Expression and purification of Canis interferon α in Escherichia coli using different tags.Protein Expr Purif. 2015 Nov;115:76-82. doi: 10.1016/j.pep.2015.07.007. Epub 2015 Jul 18. Protein Expr Purif. 2015. PMID: 26196501
-
Purification of high yields of catalytically active lysyl oxidase directly from Escherichia coli cell culture.Protein Expr Purif. 2010 Nov;74(1):116-21. doi: 10.1016/j.pep.2010.06.013. Epub 2010 Jun 23. Protein Expr Purif. 2010. PMID: 20600936
-
Copper, lysyl oxidase, and extracellular matrix protein cross-linking.Am J Clin Nutr. 1998 May;67(5 Suppl):996S-1002S. doi: 10.1093/ajcn/67.5.996S. Am J Clin Nutr. 1998. PMID: 9587142 Review.
-
Stereochemistry and cofactor identity status of semicarbazide-sensitive amine oxidases.Prog Brain Res. 1995;106:41-7. doi: 10.1016/s0079-6123(08)61200-5. Prog Brain Res. 1995. PMID: 8584672 Review.
Cited by
-
Effect of Lysyl Oxidase G473 A Polymorphism on Lysyl Oxidase and Total Soluble Collagen Expression in Oral Submucous Fibrosis.Asian Pac J Cancer Prev. 2021 Aug 1;22(8):2493-2499. doi: 10.31557/APJCP.2021.22.8.2493. Asian Pac J Cancer Prev. 2021. PMID: 34452563 Free PMC article.
-
Identification of Histidine 303 as the Catalytic Base of Lysyl Oxidase via Site-Directed Mutagenesis.Protein J. 2018 Feb;37(1):47-57. doi: 10.1007/s10930-017-9749-3. Protein J. 2018. PMID: 29127553
-
In Vitro Oxidative Crosslinking of Recombinant Barnacle Cyprid Cement Gland Proteins.Mar Biotechnol (NY). 2021 Dec;23(6):928-942. doi: 10.1007/s10126-021-10076-x. Epub 2021 Oct 29. Mar Biotechnol (NY). 2021. PMID: 34714445 Free PMC article.
-
Study of Class I and Class III Polyhydroxyalkanoate (PHA) Synthases with Substrates Containing a Modified Side Chain.Biomacromolecules. 2016 Apr 11;17(4):1477-85. doi: 10.1021/acs.biomac.6b00082. Epub 2016 Mar 22. Biomacromolecules. 2016. PMID: 26974339 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

