Hypoxia induces macrophage polarization and re-education toward an M2 phenotype in U87 and U251 glioblastoma models
- PMID: 26942063
- PMCID: PMC4760330
- DOI: 10.1080/2162402X.2015.1056442
Hypoxia induces macrophage polarization and re-education toward an M2 phenotype in U87 and U251 glioblastoma models
Abstract
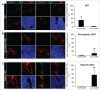
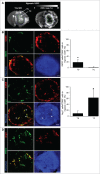
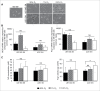
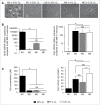
Hypoxia is a common feature of solid tumors, particularly in glioblastoma (GBM), and known to be a poor prognosis factor in GBM patients. The growth of GBM is also associated with a marked inflammation partially characterized by an accumulation of macrophage (MΦ) of the M2 phenotype. However, the transition between M1 MΦ (antitumoral) and M2 MΦ (protumoral) phenotypes is a dynamic process. We made the assumption that oxygen (O2) availability could be a major regulator of this transition and that the intratumoral O2 gradient is of importance. We evaluated, in vivo, the impact of hypoxia on MΦ tropism and polarization in two models of human GBM, well differentiated by their degree of hypoxia. MΦ migration in the tumor was more pronounced in the more hypoxic tumor of the two GBM models. In the more hypoxic of the models, we have shown that MΦ migrated at the tumor site only when hypoxia takes place. We also demonstrated that the acquisition of the M2 phenotype was clearly an evolving phenomenon with hypoxia as the major trigger for this transition. In support of these in vivo finding, M0 but also M1 MΦ cultured in moderate or severe hypoxia displayed a phenotype close to that of M2 MΦ whose phenotype was further reinforced by severe hypoxia. These results highlight the role of hypoxia in the aggressiveness of GBM, in part, by transforming MΦ such that a protumoral activity is expressed.
Keywords: M2 macrophages; brain tumor; hypoxia; polarization; re-education.
Figures


References
-
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJB, Belanger K, Brandes A, Marosi C, Bogdahn U et al.. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005; 352:987-96; PMID:15758009; http://dx.doi.org/10.1056/NEJMoa043330 - DOI - PubMed
-
- Charles NA, Holland EC, Gilbertson R, Glass R, Kettenmann H. The brain tumor microenvironment. Glia 2011; 59:1169-80; PMID:21446047; http://dx.doi.org/10.1002/glia.21136 - DOI - PubMed
-
- Clavreul A, Etcheverry A, Chassevent A, Quillien V, Avril T, Jourdan M-L, Michalak S, François P, Carré J-L, Mosser J et al.. Isolation of a new cell population in the glioblastoma microenvironment. J Neurooncol 2012; 106:493-504; PMID:21928115; http://dx.doi.org/10.1007/s11060-011-0701-7 - DOI - PubMed
-
- Hirata K, Terasaka S, Shiga T, Hattori N, Magota K, Kobayashi H, Yamaguchi S, Houkin K, Tanaka S, Kuge Y et al.. 18F-Fluoromisonidazole positron emission tomography may differentiate glioblastoma multiforme from less malignant gliomas. Eur J Nucl Med Mol Imaging 2012; 39:760-70; PMID:22307533; http://dx.doi.org/10.1007/s00259-011-2037-0 - DOI - PubMed
-
- Rampling R, Cruickshank G, Lewis AD, Fitzsimmons SA, Workman P. Direct measurement of pO2 distribution and bioreductive enzymes in human malignant brain tumors. Int J Radiat Oncol Biol Phys 1994; 29:427-31; PMID:8005794; http://dx.doi.org/10.1016/0360-3016(94)90432-4 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
