Ibrutinib enhances IL-17 response by modulating the function of bone marrow derived dendritic cells
- PMID: 26942065
- PMCID: PMC4760285
- DOI: 10.1080/2162402X.2015.1057385
Ibrutinib enhances IL-17 response by modulating the function of bone marrow derived dendritic cells
Abstract
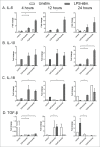
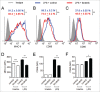
Ibrutinib (PCI-32765) is an irreversible dual Btk/Itk inhibitor shown to be effective in treating several B cell malignancies. However, limited studies have been conducted to study the effect of this drug on myeloid cell function. Hence, we studied the effect of ibrutinib treatment on TLR-4 mediated activation of bone marrow derived dendritic cell culture (DCs). Upon ibrutinib treatment, LPS-treated DCs displayed lower synthesis of TNF-α and nitric oxide (NO) and higher induction of IL-6, TGF-β, IL-10 and IL-18. While ibrutinib dampened MHC-II and CD86 expression on DCs, CD80 expression was upregulated. Further, ibrutinib-treated DCs promoted T cell proliferation and enhanced IL-17 production upon co-culture with nylon wool enriched T cells. Taken together, our results indicate that ibrutinib modulates TLR-4 mediated DC activation to promote an IL-17 response. We describe a novel mode of action for ibrutinib on DCs which should be explored to treat other forms of cancer besides B cell malignancies.
Keywords: Btk; IL-17; T cells; TLR-4; dendritic cells; ibrutinib.
Figures


References
-
- Honigberg LA, Smith AM, Sirisawad M, Verner E, Loury D, Chang B, Li S, Pan Z, Thamm DH, Miller RA et al.. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc Natl Acad Sci U S A 2010; 107:13075-80; PMID:20615965; http://dx.doi.org/10.1073/pnas.1004594107 - DOI - PMC - PubMed
-
- Dubovsky JA, Beckwith KA, Natarajan G, Woyach J, Jaglowski S, Zhong Y, Hessler JD, Liu T-M, Chang BY, Larkin KM et al.. Ibrutinib is an irreversible molecular inhibitor of ITK driving a Th1-selective pressure in T lymphocytes. Blood 2013; 122:2539-49; PMID:23886836; http://dx.doi.org/10.1182/blood-2013-06-507947 - DOI - PMC - PubMed
-
- Akinleye A, Chen Y, Mukhi N, Song Y, Liu D. Ibrutinib and novel BTK inhibitors in clinical development. J Hematol Oncol 2013; 6:59; PMID:23958373; http://dx.doi.org/10.1186/1756-8722-6-59 - DOI - PMC - PubMed
-
- Maddocks K, Blum KA. Ibrutinib in B-cell Lymphomas. Curr Treat Options Oncol 2014; 15:226-37; PMID:24481980; http://dx.doi.org/10.1007/s11864-014-0274-8 - DOI - PubMed
-
- Burger JA, Buggy JJ. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765). Leuk Lymphoma 2013; 54:2385-91; PMID:23425038; http://dx.doi.org/10.3109/10428194.2013.777837 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
