Comparison of naïve and central memory derived CD8+ effector cell engraftment fitness and function following adoptive transfer
- PMID: 26942092
- PMCID: PMC4760301
- DOI: 10.1080/2162402X.2015.1072671
Comparison of naïve and central memory derived CD8+ effector cell engraftment fitness and function following adoptive transfer
Abstract
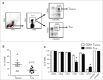
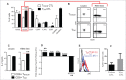
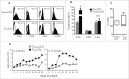
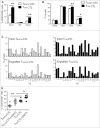
Human CD8+ effector T cells derived from CD45RO+CD62L+ precursors enriched for central memory (TCM) precursors retain the capacity to engraft and reconstitute functional memory upon adoptive transfer, whereas effectors derived from CD45RO+CD62L- precursors enriched for effector memory precursors do not. Here we sought to compare the engraftment fitness and function of CD8+ effector T cells derived from CD45RA+CD62L+ precursors enriched for naïve and stem cell memory precursors (TN/SCM) with that of TCM. We found that cytotoxic T cells (CTLs) derived from TCM transcribed higher levels of CD28, FOS, INFγ, Eomesodermin (Eomes), and lower levels of BCL2L11, maintained higher levels of phosphorylated AKT, and displayed enhanced sensitivity to the proliferative and anti-apoptotic effects of γ-chain cytokines compared to CTLs derived from TN/SCM. Higher frequencies of CTLs derived from TCM retained CD28 expression and upon activation secreted higher levels of IL-2. In NOD/Scid IL-2RγCnull mice, CD8+ TCM derived CTLs engrafted to higher frequencies in response to human IL-15 and mounted robust proliferative responses to an immunostimulatory vaccine. Similarly, CD8+ TCM derived CD19CAR+ CTLs exhibited superior antitumor potency following adoptive transfer compared to their CD8+ TN/SCM derived counterparts. These studies support the use of TCM enriched cell products for adoptive therapy of cancer.
Keywords: Antitumor activity; adoptive therapy; central memory T cell derived CTLs; engraftment fitness; naïve T cell derived CTLs.
Figures

Similar articles
-
Phenotypic and functional attributes of lentivirus-modified CD19-specific human CD8+ central memory T cells manufactured at clinical scale.J Immunother. 2012 Nov-Dec;35(9):689-701. doi: 10.1097/CJI.0b013e318270dec7. J Immunother. 2012. PMID: 23090078 Free PMC article.
-
Engraftment of human central memory-derived effector CD8+ T cells in immunodeficient mice.Blood. 2011 Feb 10;117(6):1888-98. doi: 10.1182/blood-2010-10-310599. Epub 2010 Dec 1. Blood. 2011. PMID: 21123821 Free PMC article.
-
Variances in Antiviral Memory T-Cell Repertoire of CD45RA- and CD62L-Depleted Lymphocyte Products Reflect the Need of Individual T-Cell Selection Strategies to Reduce the Risk of GvHD while Preserving Antiviral Immunity in Adoptive T-Cell Therapy.Transfus Med Hemother. 2021 Sep 10;49(1):30-43. doi: 10.1159/000516284. eCollection 2022 Feb. Transfus Med Hemother. 2021. PMID: 35221866 Free PMC article.
-
In vitro generated anti-tumor T lymphocytes exhibit distinct subsets mimicking in vivo antigen-experienced cells.Cancer Immunol Immunother. 2011 May;60(5):739-49. doi: 10.1007/s00262-011-0977-7. Epub 2011 Feb 9. Cancer Immunol Immunother. 2011. PMID: 21305379 Free PMC article.
-
Human effector T cells derived from central memory cells rather than CD8(+)T cells modified by tumor-specific TCR gene transfer possess superior traits for adoptive immunotherapy.Cancer Lett. 2013 Oct 10;339(2):195-207. doi: 10.1016/j.canlet.2013.06.009. Epub 2013 Jun 18. Cancer Lett. 2013. PMID: 23791878
Cited by
-
Next-day manufacture of a novel anti-CD19 CAR-T therapy for B-cell acute lymphoblastic leukemia: first-in-human clinical study.Blood Cancer J. 2022 Jul 7;12(7):104. doi: 10.1038/s41408-022-00694-6. Blood Cancer J. 2022. PMID: 35798714 Free PMC article. Clinical Trial.
-
Improving CAR T-Cell Persistence.Int J Mol Sci. 2021 Oct 7;22(19):10828. doi: 10.3390/ijms221910828. Int J Mol Sci. 2021. PMID: 34639168 Free PMC article. Review.
-
Drug-regulated CD33-targeted CAR T cells control AML using clinically optimized rapamycin dosing.J Clin Invest. 2024 Mar 19;134(9):e162593. doi: 10.1172/JCI162593. J Clin Invest. 2024. PMID: 38502193 Free PMC article. Clinical Trial.
-
Ex vivo enrichment of PRAME antigen-specific T cells for adoptive immunotherapy using CD137 activation marker selection.Clin Transl Immunology. 2020 Oct 21;9(10):e1200. doi: 10.1002/cti2.1200. eCollection 2020. Clin Transl Immunology. 2020. PMID: 33101678 Free PMC article.
-
Reducing Ex Vivo Culture Improves the Antileukemic Activity of Chimeric Antigen Receptor (CAR) T Cells.Cancer Immunol Res. 2018 Sep;6(9):1100-1109. doi: 10.1158/2326-6066.CIR-17-0405. Epub 2018 Jul 20. Cancer Immunol Res. 2018. PMID: 30030295 Free PMC article. Clinical Trial.
References
-
- Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, Teachey DT, Chew A, Hauck B, Wright JF et al.. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med 2013; 368:1509-18; PMID:23527958; http://dx.doi.org/10.1056/NEJMoa1215134 - DOI - PMC - PubMed
-
- Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, June CH. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med 2011; 3:95ra73; PMID:21832238; http://dx.doi.org/10.1126/scitranslmed.3002842 - DOI - PMC - PubMed
-
- Brentjens RJ, Davila ML, Riviere I, Park J, Wang X, Cowell LG, Bartido S, Stefanski J, Taylor C, Olszewska M et al.. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med 2013; 5:177ra38; PMID:23515080; http://dx.doi.org/10.1126/scitranslmed.3005930 - DOI - PMC - PubMed
-
- Kochenderfer JN, Dudley ME, Feldman SA, Wilson WH, Spaner DE, Maric I, Stetler-Stevenson M, Phan GQ, Hughes MS, Sherry RM et al.. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood 2012; 119:2709-20; PMID:22160384; http://dx.doi.org/10.1182/blood-2011-10-384388 - DOI - PMC - PubMed
-
- Wang X, Berger C, Wong CW, Forman SJ, Riddell SR, Jensen MC. Engraftment of human central memory-derived effector CD8+ T cells in immunodeficient mice. Blood 2011; 117:1888-98; PMID:21123821; http://dx.doi.org/10.1182/blood-2010-10-310599 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
