Loss of VPS13C Function in Autosomal-Recessive Parkinsonism Causes Mitochondrial Dysfunction and Increases PINK1/Parkin-Dependent Mitophagy
- PMID: 26942284
- PMCID: PMC4800038
- DOI: 10.1016/j.ajhg.2016.01.014
Loss of VPS13C Function in Autosomal-Recessive Parkinsonism Causes Mitochondrial Dysfunction and Increases PINK1/Parkin-Dependent Mitophagy
Abstract
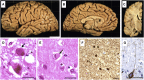
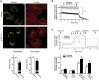
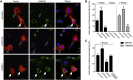
Autosomal-recessive early-onset parkinsonism is clinically and genetically heterogeneous. The genetic causes of approximately 50% of autosomal-recessive early-onset forms of Parkinson disease (PD) remain to be elucidated. Homozygozity mapping and exome sequencing in 62 isolated individuals with early-onset parkinsonism and confirmed consanguinity followed by data mining in the exomes of 1,348 PD-affected individuals identified, in three isolated subjects, homozygous or compound heterozygous truncating mutations in vacuolar protein sorting 13C (VPS13C). VPS13C mutations are associated with a distinct form of early-onset parkinsonism characterized by rapid and severe disease progression and early cognitive decline; the pathological features were striking and reminiscent of diffuse Lewy body disease. In cell models, VPS13C partly localized to the outer membrane of mitochondria. Silencing of VPS13C was associated with lower mitochondrial membrane potential, mitochondrial fragmentation, increased respiration rates, exacerbated PINK1/Parkin-dependent mitophagy, and transcriptional upregulation of PARK2 in response to mitochondrial damage. This work suggests that loss of function of VPS13C is a cause of autosomal-recessive early-onset parkinsonism with a distinctive phenotype of rapid and severe progression.
Copyright © 2016 The American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Figures



Comment in
-
VPS13C-Another Hint at Mitochondrial Dysfunction in Familial Parkinson's Disease.Mov Disord. 2016 Sep;31(9):1340. doi: 10.1002/mds.26682. Epub 2016 May 23. Mov Disord. 2016. PMID: 27213732 No abstract available.
References
-
- Bonifati V. Genetics of Parkinson’s disease--state of the art, 2013. Parkinsonism Relat. Disord. 2014;20(Suppl 1):S23–S28. - PubMed
-
- Lücking C.B., Dürr A., Bonifati V., Vaughan J., De Michele G., Gasser T., Harhangi B.S., Meco G., Denèfle P., Wood N.W., French Parkinson’s Disease Genetics Study Group. European Consortium on Genetic Susceptibility in Parkinson’s Disease Association between early-onset Parkinson’s disease and mutations in the parkin gene. N. Engl. J. Med. 2000;342:1560–1567. - PubMed
-
- Ibáñez P., Lesage S., Lohmann E., Thobois S., De Michele G., Borg M., Agid Y., Dürr A., Brice A., French Parkinson’s Disease Genetics Study Group Mutational analysis of the PINK1 gene in early-onset parkinsonism in Europe and North Africa. Brain. 2006;129:686–694. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

