The Value of In Vitro Diagnostic Testing in Medical Practice: A Status Report
- PMID: 26942417
- PMCID: PMC4778800
- DOI: 10.1371/journal.pone.0149856
The Value of In Vitro Diagnostic Testing in Medical Practice: A Status Report
Erratum in
-
Correction: The Value of In Vitro Diagnostic Testing in Medical Practice: A Status Report.PLoS One. 2016 Apr 14;11(4):e0154008. doi: 10.1371/journal.pone.0154008. eCollection 2016. PLoS One. 2016. PMID: 27078838 Free PMC article. No abstract available.
Abstract
Background: In vitro diagnostic (IVD) investigations are indispensable for routine patient management. Appropriate testing allows early-stage interventions, reducing late-stage healthcare expenditure (HCE).
Aim: To investigate HCE on IVDs in two developed markets and to assess the perceived value of IVDs on clinical decision-making. Physician-perceived HCE on IVD was evaluated, as well as desired features of new diagnostic markers.
Methods: Past and current HCE on IVD was calculated for the US and Germany. A total of 79 US/German oncologists and cardiologists were interviewed to assess the number of cases where: physicians ask for IVDs; IVDs are used for initial diagnosis, treatment monitoring, or post-treatment; and decision-making is based on an IVD test result. A sample of 201 US and German oncologists and cardiologists was questioned regarding the proportion of HCE they believed to be attributable to IVD testing. After disclosing the actual IVD HCE, the physician's perception of the appropriateness of the amount was captured. Finally, the association between physician-rated impact of IVD on decision-making and perceived contribution of IVD expenditure on overall HCE was assessed.
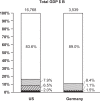
Results: IVD costs account for 2.3% and 1.4% of total HCE in the US and Germany. Most physicians (81%) believed that the actual HCE on IVDs was >5%; 19% rated the spending correctly (0-4%, p<0.001). When informed of the actual amount, 64% of physicians rated this as appropriate (p<0.0001); 66% of decision-making was based on IVD. Significantly, more physicians asked for either additional clinical or combined clinical/health economic data than for the product (test/platform) alone (p<0.0001).
Conclusions: Our results indicate a poor awareness of actual HCE on IVD, but a high attributable value of diagnostic procedures for patient management. New markers should deliver actionable and medically relevant information, to guide decision-making and foster improved patient outcomes.
Conflict of interest statement
Figures


References
-
- Raman G, Avendano E, Chen M. Update on Emerging Genetic Tests Currently Available for Clinical Use in Common Cancers, in Technology Assessment Report Rockville, MD: Agency for Healthcare Research and Quality; 2013. - PubMed
-
- Billings PR. Three barriers to innovative diagnostics. Nat Biotechnol. 2006;24:917–918. - PubMed
-
- Blendon RJ, Schoen C, DesRoches CM, Osborn R, Zapert K, Raleigh E. Confronting competing demands to improve quality: a five-country hospital survey. Health Aff (Millwood). 2004;23:119–135. - PubMed
-
- Wilke MH, Schenker M, Hoffmann G. Detection and documentation of DRG-relevant comorbidities using laboratory tests. Aust Health Rev. 2002;25:152–160. - PubMed
-
- Peto J, Gilham C, Fletcher O, Matthews FE. The cervical cancer epidemic that screening has prevented in the UK. Lancet. 2004;364:249–256. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

