Overexpression of RPS27a contributes to enhanced chemoresistance of CML cells to imatinib by the transactivated STAT3
- PMID: 26942564
- PMCID: PMC4951316
- DOI: 10.18632/oncotarget.7888
Overexpression of RPS27a contributes to enhanced chemoresistance of CML cells to imatinib by the transactivated STAT3
Abstract
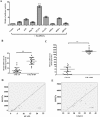
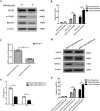
STAT3 plays a pivotal role in the hematopoietic system, which constitutively activated by BCR-ABL via JAK and Erk/MAP-kinase pathways. Phospho-STAT3 was overexpressed in imatinib-resistant CML patients as relative to imatinib responsive ones. By activation of the STAT3 pathway, BCR-ABL can promote cell cycling, and inhibit differentiation and apoptosis. Ribosomal protein S27a (RPS27a) performs extra-ribosomal functions besides imparting a role in ribosome biogenesis and post-translational modifications of proteins. RPS27a can promote proliferation, regulate cell cycle progression and inhibit apoptosis of leukemia cells. However, the relationship between STAT3 and RPS27a has not been reported. In this study, we detected a significantly increased expression of STAT3 and RPS27a in bone marrow samples from CML-AP/BP patients compared with those from CML-CP. In addition, we also demonstrated that it was a positive correlation between the level of STAT3 and that of RPS27a. Imatinib-resistant K562/G01 cells expressed significantly higher levels of STAT3 and RPS27a compared with those of K562 cells. RPS27a could be transactivated by p-STAT3 through the specific p-STAT3-binding site located nt -633 to -625 and -486 to -478 of the RPS27a gene promoter in a dose-dependent manner. The transactivated RPS27a could decrease the percentage of apoptotic CML cells induced by imatinib. And the effect of STAT3 overexpression could be counteracted by the p-STAT3 inhibitor WP1066 or RPS27a knockdown. These results suggest that drugs targeting STAT3/p-STAT3/RPS27a combining with TKI might represent a novel therapy strategy in patients with TKI-resistant CML.
Keywords: CML; RPS27a; STAT3; apoptosis; imatinib.
Conflict of interest statement
The authors declare no financial or other conflicts of interest.
Figures




Similar articles
-
RPS27a promotes proliferation, regulates cell cycle progression and inhibits apoptosis of leukemia cells.Biochem Biophys Res Commun. 2014 Apr 18;446(4):1204-10. doi: 10.1016/j.bbrc.2014.03.086. Epub 2014 Mar 26. Biochem Biophys Res Commun. 2014. PMID: 24680683
-
Re-Expression of Bone Marrow Proteoglycan-2 by 5-Azacytidine is associated with STAT3 Inactivation and Sensitivity Response to Imatinib in Resistant CML Cells.Asian Pac J Cancer Prev. 2018 Jun 25;19(6):1585-1590. doi: 10.22034/APJCP.2018.19.6.1585. Asian Pac J Cancer Prev. 2018. PMID: 29936783 Free PMC article.
-
Targeting Hedgehog signaling pathway and autophagy overcomes drug resistance of BCR-ABL-positive chronic myeloid leukemia.Autophagy. 2015;11(2):355-72. doi: 10.4161/15548627.2014.994368. Autophagy. 2015. PMID: 25701353 Free PMC article.
-
Novel targeted therapies to overcome imatinib mesylate resistance in chronic myeloid leukemia (CML).Crit Rev Oncol Hematol. 2006 Feb;57(2):145-64. doi: 10.1016/j.critrevonc.2005.06.007. Epub 2005 Oct 5. Crit Rev Oncol Hematol. 2006. PMID: 16213151 Review.
-
Regulating Apoptosis by Degradation: The N-End Rule-Mediated Regulation of Apoptotic Proteolytic Fragments in Mammalian Cells.Int J Mol Sci. 2018 Oct 31;19(11):3414. doi: 10.3390/ijms19113414. Int J Mol Sci. 2018. PMID: 30384441 Free PMC article. Review.
Cited by
-
Mitochondrial Ribosomal Protein L14 Promotes Cell Growth and Invasion by Modulating Reactive Oxygen Species in Thyroid Cancer.Clin Exp Otorhinolaryngol. 2023 May;16(2):184-197. doi: 10.21053/ceo.2022.01760. Epub 2023 Feb 23. Clin Exp Otorhinolaryngol. 2023. PMID: 36822197 Free PMC article.
-
Case Report: Molecular and microenvironment change upon midostaurin treatment in mast cell leukemia at single-cell level.Front Immunol. 2023 Aug 10;14:1210909. doi: 10.3389/fimmu.2023.1210909. eCollection 2023. Front Immunol. 2023. PMID: 37638009 Free PMC article.
-
Decreased expression of LncRNA SLC25A25-AS1 promotes proliferation, chemoresistance, and EMT in colorectal cancer cells.Tumour Biol. 2016 Oct;37(10):14205-14215. doi: 10.1007/s13277-016-5254-0. Epub 2016 Aug 23. Tumour Biol. 2016. PMID: 27553025
-
Silver Nanoparticles Derived by Artemisia arborescens Reveal Anticancer and Apoptosis-Inducing Effects.Int J Mol Sci. 2021 Aug 11;22(16):8621. doi: 10.3390/ijms22168621. Int J Mol Sci. 2021. PMID: 34445327 Free PMC article.
-
Sulfasalazine synergistically enhances the inhibitory effects of imatinib against hepatocellular carcinoma (HCC) cells by targeting NFκB, BCR/ABL, and PI3K/AKT signaling pathway-related proteins.FEBS Open Bio. 2021 Mar;11(3):588-597. doi: 10.1002/2211-5463.13052. Epub 2021 Feb 20. FEBS Open Bio. 2021. PMID: 33289342 Free PMC article.
References
-
- Deininger MW, Goldman JM, Melo JV. The molecular biology of chronic myeloid leukemia. Blood. 2000;96:3343–3356. - PubMed
-
- Sattler M, Griffin JD. Molecular mechanisms of transformation by the BCR-ABL oncogene. Semin Hematol. 2003;40:4–10. - PubMed
-
- Mauro MJ, O'Dwyer ME, Druker BJ. ST1571, a tyrosine kinase inhibitor for the treatment of chronic myelogenous leukemia: validating the promise of molecularly targeted therapy. Cancer Chemother Pharmacol. 2001;48:S77–78. - PubMed
-
- Druker BJ, Talpaz M, Resta DJ, Peng B, Buchdunger E, Ford JM, Lydon NB, Kantarjian H, Capdeville R, Ohno-Jones S, Sawyers CL. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031–1037. - PubMed
-
- Kantarjian HM, Cortes JE, O'Brien S, Giles F, Garcia-Manero G, Faderl S, Thomas D, Jeha S, Rios MB, Letvak L, Bochinski K, Arlinghaus R, Talpaz M. Imatinib mesylate therapy in newly diagnosed patients with Philadelphia chromosome-positive chronic myelogenous leukemia: high incidence of early complete and major cytogenetic responses. Blood. 2003;101:97–100. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

