Overexpression of RPS27a contributes to enhanced chemoresistance of CML cells to imatinib by the transactivated STAT3
- PMID: 26942564
- PMCID: PMC4951316
- DOI: 10.18632/oncotarget.7888
Overexpression of RPS27a contributes to enhanced chemoresistance of CML cells to imatinib by the transactivated STAT3
Abstract
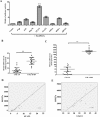
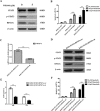
STAT3 plays a pivotal role in the hematopoietic system, which constitutively activated by BCR-ABL via JAK and Erk/MAP-kinase pathways. Phospho-STAT3 was overexpressed in imatinib-resistant CML patients as relative to imatinib responsive ones. By activation of the STAT3 pathway, BCR-ABL can promote cell cycling, and inhibit differentiation and apoptosis. Ribosomal protein S27a (RPS27a) performs extra-ribosomal functions besides imparting a role in ribosome biogenesis and post-translational modifications of proteins. RPS27a can promote proliferation, regulate cell cycle progression and inhibit apoptosis of leukemia cells. However, the relationship between STAT3 and RPS27a has not been reported. In this study, we detected a significantly increased expression of STAT3 and RPS27a in bone marrow samples from CML-AP/BP patients compared with those from CML-CP. In addition, we also demonstrated that it was a positive correlation between the level of STAT3 and that of RPS27a. Imatinib-resistant K562/G01 cells expressed significantly higher levels of STAT3 and RPS27a compared with those of K562 cells. RPS27a could be transactivated by p-STAT3 through the specific p-STAT3-binding site located nt -633 to -625 and -486 to -478 of the RPS27a gene promoter in a dose-dependent manner. The transactivated RPS27a could decrease the percentage of apoptotic CML cells induced by imatinib. And the effect of STAT3 overexpression could be counteracted by the p-STAT3 inhibitor WP1066 or RPS27a knockdown. These results suggest that drugs targeting STAT3/p-STAT3/RPS27a combining with TKI might represent a novel therapy strategy in patients with TKI-resistant CML.
Keywords: CML; RPS27a; STAT3; apoptosis; imatinib.
Conflict of interest statement
The authors declare no financial or other conflicts of interest.
Figures




References
-
- Deininger MW, Goldman JM, Melo JV. The molecular biology of chronic myeloid leukemia. Blood. 2000;96:3343–3356. - PubMed
-
- Sattler M, Griffin JD. Molecular mechanisms of transformation by the BCR-ABL oncogene. Semin Hematol. 2003;40:4–10. - PubMed
-
- Mauro MJ, O'Dwyer ME, Druker BJ. ST1571, a tyrosine kinase inhibitor for the treatment of chronic myelogenous leukemia: validating the promise of molecularly targeted therapy. Cancer Chemother Pharmacol. 2001;48:S77–78. - PubMed
-
- Druker BJ, Talpaz M, Resta DJ, Peng B, Buchdunger E, Ford JM, Lydon NB, Kantarjian H, Capdeville R, Ohno-Jones S, Sawyers CL. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031–1037. - PubMed
-
- Kantarjian HM, Cortes JE, O'Brien S, Giles F, Garcia-Manero G, Faderl S, Thomas D, Jeha S, Rios MB, Letvak L, Bochinski K, Arlinghaus R, Talpaz M. Imatinib mesylate therapy in newly diagnosed patients with Philadelphia chromosome-positive chronic myelogenous leukemia: high incidence of early complete and major cytogenetic responses. Blood. 2003;101:97–100. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

