High-throughput drug library screening identifies colchicine as a thyroid cancer inhibitor
- PMID: 26942566
- PMCID: PMC4991430
- DOI: 10.18632/oncotarget.7890
High-throughput drug library screening identifies colchicine as a thyroid cancer inhibitor
Abstract
We employed a high-throughput drug library screening platform to identify novel agents affecting thyroid cancer cells. We used human thyroid cancer cell lines to screen a collection of approximately 5200 small molecules with biological and/or pharmacologial properties. Parallel primary screens yielded a number of hits differentially active between thyroid and melanoma cells. Amongst compounds specifically targeting thyroid cancer cells, colchicine emerged as an effective candidate. Colchicine inhibited cell growth which correlated with G2 cell cycle arrest and apoptosis. These effects were hampered through inhibition of MEK1/2 and JNK. In contrast, inhibition of p38-MAPK had little effect, and AKT had no impact on colchicine action. Systemic colchicine inhibited thyroid cancer progression in xenografted mice. These findings demonstrate that our screening platform is an effective vehicle for drug reposition and show that colchicine warrants further attention in well-defined clinical niches such as thyroid cancer.
Keywords: BRAF; BRAF resistance; colchicine; high throughput drug screening; thyroid cancer.
Conflict of interest statement
The authors disclose no potential conflicts of interest.
Figures
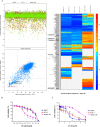
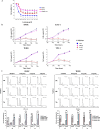
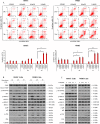

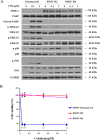
References
-
- Dickson MA, Gordon MS, Edelman G, Bendell JC, Kudchadkar RR, LoRusso PM, Johnston SH, Clary DO, Schwartz GK. Phase I study of XL281 (BMS-908662), a potent oral RAF kinase inhibitor, in patients with advanced solid tumors. Invest New Drugs. 2015;33:349–356. - PubMed
-
- Ahronian LG, Sennott EM, Van Allen EM, Wagle N, Kwak EL, Faris JE, Godfrey JT, Nishimura K, Lynch KD, Mermel CH, Lockerman EL, Kalsy A, Gurski JM, et al. Clinical Acquired Resistance to RAF Inhibitor Combinations in BRAF-Mutant Colorectal Cancer through MAPK Pathway Alterations. Cancer Discov. 2015;5:358–367. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

