Deletion of the γ-secretase subunits Aph1B/C impairs memory and worsens the deficits of knock-in mice modeling the Alzheimer-like familial Danish dementia
- PMID: 26942869
- PMCID: PMC4914259
- DOI: 10.18632/oncotarget.7389
Deletion of the γ-secretase subunits Aph1B/C impairs memory and worsens the deficits of knock-in mice modeling the Alzheimer-like familial Danish dementia
Abstract
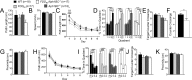
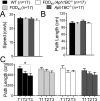
Mutations in BRI2/ITM2b genes cause Familial British and Danish Dementias (FBD and FDD), which are pathogenically similar to Familial Alzheimer Disease (FAD). BRI2 inhibits processing of Amyloid precursor protein (APP), a protein involved in FAD pathogenesis. Accumulation of a carboxyl-terminal APP metabolite -ß-CTF- causes memory deficits in a knock-in mouse model of FDD, called FDDKI.We have investigated further the pathogenic function of ß-CTF studying the effect of Aph1B/C deletion on FDDKI mice. This strategy is based on the evidence that deletion of Aph1B/C proteins, which are components of the γ-secretase that cleaves ß-CTF, results in stabilization of ß-CTF and a reduction of Aβ. We found that both the FDD mutation and the Aph1B/C deficiency mildly interfered with spatial long term memory, spatial working/short-term memory and long-term contextual fear memory. In addition, the Aph1BC deficiency induced deficits in long-term cued fear memory. Moreover, the two mutations have additive adverse effects as they compromise the accuracy of spatial long-term memory and induce spatial memory retention deficits in young mice. Overall, the data are consistent with a role for β-CTF in the genesis of memory deficits.
Keywords: APP; Alzheimer; Danish dementia; Gerotarget; Itm2b-bri2; gamma-secretase.
Conflict of interest statement
The AECOM has a patent on the commercial use of FDDKI mice. Luciano D'Adamio is a co-inventor on this patent. AECOM has licensed the patent to Remegenix, a company of which Luciano D'Adamio is a co-founder and a Board member. As a co-founder Luciano D'Adamio owns 35% of Remegenix. The patent and the licensing only covers commercial use of the mice and does not pose any obstacle to distribution of the mice to academic laboratories. There are no further patents, products in development or marketed products to declare. This does not alter the authors' adherence to all the Oncotarget's policies on sharing data and materials, as detailed online in the guide for authors.
Figures











References
-
- Matsuda S, Giliberto L, Matsuda Y, Davies P, McGowan E, Pickford F, Ghiso J, Frangione B, D'Adamio L. The familial dementia BRI2 gene binds the Alzheimer gene amyloid-beta precursor protein and inhibits amyloid-beta production. J Biol Chem. 2005;280:28912–28916. - PubMed
-
- Vidal R, Frangione B, Rostagno A, Mead S, Revesz T, Plant G, Ghiso J. A stop-codon mutation in the BRI gene associated with familial British dementia. Nature. 1999;399:776–781. - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

