Deprescribing in Frail Older People: A Randomised Controlled Trial
- PMID: 26942907
- PMCID: PMC4778763
- DOI: 10.1371/journal.pone.0149984
Deprescribing in Frail Older People: A Randomised Controlled Trial
Abstract
Objectives: Deprescribing has been proposed as a way to reduce polypharmacy in frail older people. We aimed to reduce the number of medicines consumed by people living in residential aged care facilities (RACF). Secondary objectives were to explore the effect of deprescribing on survival, falls, fractures, hospital admissions, cognitive, physical, and bowel function, quality of life, and sleep.
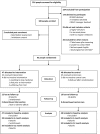
Methods: Ninety-five people aged over 65 years living in four RACF in rural mid-west Western Australia were randomised in an open study. The intervention group (n = 47) received a deprescribing intervention, the planned cessation of non-beneficial medicines. The control group (n = 48) received usual care. Participants were monitored for twelve months from randomisation. Primary outcome was change in the mean number of unique regular medicines. All outcomes were assessed at baseline, six, and twelve months.
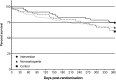
Results: Study participants had a mean age of 84.3 ± 6.9 years and 52% were female. Intervention group participants consumed 9.6 ± 5.0 and control group participants consumed 9.5 ± 3.6 unique regular medicines at baseline. Of the 348 medicines targeted for deprescribing (7.4 ± 3.8 per person, 78% of regular medicines), 207 medicines (4.4 ± 3.4 per person, 59% of targeted medicines) were successfully discontinued. The mean change in number of regular medicines at 12 months was -1.9 ± 4.1 in intervention group participants and +0.1 ± 3.5 in control group participants (estimated difference 2.0 ± 0.9, 95%CI 0.08, 3.8, p = 0.04). Twelve intervention participants and 19 control participants died within 12 months of randomisation (26% versus 40% mortality, p = 0.16, HR 0.60, 95%CI 0.30 to 1.22) There were no significant differences between groups in other secondary outcomes. The main limitations of this study were the open design and small participant numbers.
Conclusions: Deprescribing reduced the number of regular medicines consumed by frail older people living in residential care with no significant adverse effects on survival or other clinical outcomes.
Trial registration: Australian New Zealand Clinical Trials Registry ACTRN12611000370909.
Conflict of interest statement
Figures



References
-
- Jörgensen T, Johansson S, Kennerfalk A, Wallander M-A, Svärdsudd K (2001) Prescription Drug Use, Diagnoses, and Healthcare Utilization among the Elderly. Annals of Pharmacotherapy 35: 1004–1009. - PubMed
-
- Linjakumpu T, Hartikainen S, Klaukka T, Veijola J, Kivelä S-L, et al. (2002) Use of medications and polypharmacy are increasing among the elderly. Journal of Clinical Epidemiology 55: 809–817. - PubMed
-
- Morgan TK, Williamson M, Pirotta M, Stewart K, Myers SP, et al. (2012) A national census of medicines use: a 24-hour snapshot of Australians aged 50 years and older. Med J Aust 196: 50–53. - PubMed
-
- Kaufman DW, Kelly JP, Rosenberg L, Anderson TE, Mitchell AA (2002) Recent patterns of medication use in the ambulatory adult population of the united states: The slone survey. JAMA 287: 337–344. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

