Influenza A virus enhances its propagation through the modulation of Annexin-A1 dependent endosomal trafficking and apoptosis
- PMID: 26943321
- PMCID: PMC4946891
- DOI: 10.1038/cdd.2016.19
Influenza A virus enhances its propagation through the modulation of Annexin-A1 dependent endosomal trafficking and apoptosis
Abstract
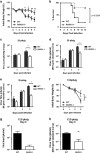
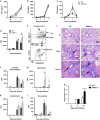
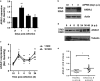
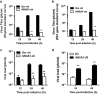
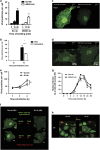
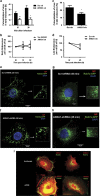
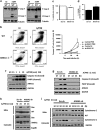
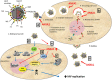
The influenza virus infects millions of people each year and can result in severe complications. Understanding virus recognition and host responses to influenza infection will enable future development of more effective anti-viral therapies. Previous research has revealed diverse yet important roles for the annexin family of proteins in modulating the course of influenza A virus (IAV) infection. However, the role of Annexin-A1 (ANXA1) in IAV infection has not been addressed. Here, we show that ANXA1 deficient mice exhibit a survival advantage, and lower viral titers after infection. This was accompanied with enhanced inflammatory cell infiltration during IAV infection. ANXA1 expression is increased during influenza infection clinically, in vivo and in vitro. The presence of ANXA1 enhances viral replication, influences virus binding, and enhances endosomal trafficking of the virus to the nucleus. ANXA1 colocalizes with early and late endosomes near the nucleus, and enhances nuclear accumulation of viral nucleoprotein. In addition, ANXA1 enhances IAV-mediated apoptosis. Overall, our study demonstrates that ANXA1 plays an important role in influenza virus replication and propagation through various mechanisms and that we predict that the regulation of ANXA1 expression during IAV infection may be a viral strategy to enhance its infectivity.
Figures


References
-
- Pandemic Influenza Preparedness and Response: A WHO Guidance Document. World Health Organization: Geneva, 2013. - PubMed
-
- Yewdell J, García-Sastre A. Influenza virus still surprises. Curr Opin Microbiol 2002; 5: 414–418. - PubMed
-
- Gerke V, Moss SE. Annexins: from structure to function. Physiol Rev 2002; 82: 331–371. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

