PACS-2 mediates the ATM and NF-κB-dependent induction of anti-apoptotic Bcl-xL in response to DNA damage
- PMID: 26943323
- PMCID: PMC5072422
- DOI: 10.1038/cdd.2016.23
PACS-2 mediates the ATM and NF-κB-dependent induction of anti-apoptotic Bcl-xL in response to DNA damage
Abstract
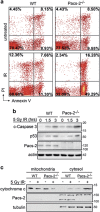
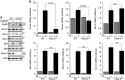
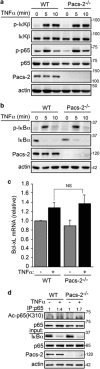
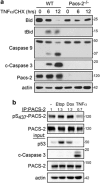
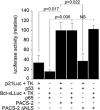
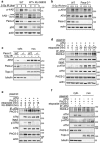
Nuclear factor kappa B (NF-κB) promotes cell survival in response to genotoxic stress by inducing the expression of anti-apoptotic proteins including Bcl-xL, which protects mitochondria from stress-induced mitochondrial outer membrane permeabilization (MOMP). Here we show that the multifunctional sorting protein Pacs-2 (phosphofurin acidic cluster sorting protein-2) is required for Bcl-xL induction following DNA damage in primary mouse thymocytes. Consequently, in response to DNA damage, Pacs-2(-/-) thymocytes exhibit a blunted induction of Bcl-xL, increased MOMP and accelerated apoptosis. Biochemical studies show that cytoplasmic PACS-2 promotes this DNA damage-induced anti-apoptotic pathway by interacting with ataxia telangiectasia mutated (ATM) to drive NF-κB activation and induction of Bcl-xL. However, Pacs-2 was not required for tumor necrosis factor-α-induced NF-κB activation, suggesting a role for PACS-2 selectively in NF-κB activation in response to DNA damage. These findings identify PACS-2 as an in vivo mediator of the ATM and NF-κB-dependent induction of Bcl-xL that promotes cell survival in response to DNA damage.
Figures

Similar articles
-
An ATM/TRIM37/NEMO Axis Counteracts Genotoxicity by Activating Nuclear-to-Cytoplasmic NF-κB Signaling.Cancer Res. 2018 Nov 15;78(22):6399-6412. doi: 10.1158/0008-5472.CAN-18-2063. Epub 2018 Sep 25. Cancer Res. 2018. PMID: 30254148
-
NF-κB activation fails to protect cells to TNFα-induced apoptosis in the absence of Bcl-xL, but not Mcl-1, Bcl-2 or Bcl-w.Biochim Biophys Acta. 2013 May;1833(5):1085-95. doi: 10.1016/j.bbamcr.2013.01.014. Epub 2013 Jan 28. Biochim Biophys Acta. 2013. PMID: 23369735
-
ATM gene regulates oxygen-glucose deprivation-induced nuclear factor-kappaB DNA-binding activity and downstream apoptotic cascade in mouse cerebrovascular endothelial cells.Stroke. 2002 Oct;33(10):2471-7. doi: 10.1161/01.str.0000030316.79601.03. Stroke. 2002. PMID: 12364740
-
The Accomplices of NF-κB Lead to Radioresistance.Curr Protein Pept Sci. 2015;16(4):279-94. doi: 10.2174/138920371604150429152328. Curr Protein Pept Sci. 2015. PMID: 25929862 Review.
-
Signalling loops and linear pathways: NF-kappaB activation in response to genotoxic stress.Mutagenesis. 2009 Jan;24(1):1-8. doi: 10.1093/mutage/gen056. Epub 2008 Oct 1. Mutagenesis. 2009. PMID: 18832076 Review.
Cited by
-
RMP/URI inhibits both intrinsic and extrinsic apoptosis through different signaling pathways.Int J Biol Sci. 2019 Oct 15;15(12):2692-2706. doi: 10.7150/ijbs.36829. eCollection 2019. Int J Biol Sci. 2019. PMID: 31754340 Free PMC article.
-
A synergistic partnership between IL-33/ST2 and Wnt pathway through Bcl-xL drives gastric cancer stemness and metastasis.Oncogene. 2023 Feb;42(7):501-515. doi: 10.1038/s41388-022-02575-5. Epub 2022 Dec 16. Oncogene. 2023. PMID: 36526851
-
7,8-Dihydroxyflavone improves neuropathological changes in the brain of Tg26 mice, a model for HIV-associated neurocognitive disorder.Sci Rep. 2021 Sep 16;11(1):18519. doi: 10.1038/s41598-021-97220-8. Sci Rep. 2021. PMID: 34531413 Free PMC article.
-
Xeroderma Pigmentosa Group A (XPA), Nucleotide Excision Repair and Regulation by ATR in Response to Ultraviolet Irradiation.Adv Exp Med Biol. 2017;996:41-54. doi: 10.1007/978-3-319-56017-5_4. Adv Exp Med Biol. 2017. PMID: 29124689 Free PMC article. Review.
-
Caught in the act - protein adaptation and the expanding roles of the PACS proteins in tissue homeostasis and disease.J Cell Sci. 2017 Jun 1;130(11):1865-1876. doi: 10.1242/jcs.199463. Epub 2017 May 5. J Cell Sci. 2017. PMID: 28476937 Free PMC article. Review.
References
-
- Thomenius MJ, Distelhorst CW. Bcl-2 on the endoplasmic reticulum: protecting the mitochondria from a distance. J Cell Sci 2003; 116(Pt 22): 4493–4499. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

