Loss of p53-mediated cell-cycle arrest, senescence and apoptosis promotes genomic instability and premature aging
- PMID: 26943586
- PMCID: PMC4914251
- DOI: 10.18632/oncotarget.7864
Loss of p53-mediated cell-cycle arrest, senescence and apoptosis promotes genomic instability and premature aging
Abstract
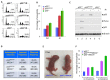
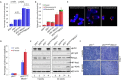
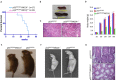
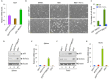
Although p53-mediated cell cycle arrest, senescence and apoptosis are well accepted as major tumor suppression mechanisms, the loss of these functions does not directly lead to tumorigenesis, suggesting that the precise roles of these canonical activities of p53 need to be redefined. Here, we report that the cells derived from the mutant mice expressing p533KR, an acetylation-defective mutant that fails to induce cell-cycle arrest, senescence and apoptosis, exhibit high levels of aneuploidy upon DNA damage. Moreover, the embryonic lethality caused by the deficiency of XRCC4, a key DNA double strand break repair factor, can be fully rescued in the p533KR/3KR background. Notably, despite high levels of genomic instability, p533KR/3KRXRCC4-/- mice, unlike p53-/- XRCC4-/- mice, are not succumbed to pro-B-cell lymphomas. Nevertheless, p533KR/3KR XRCC4-/- mice display aging-like phenotypes including testicular atrophy, kyphosis, and premature death. Further analyses demonstrate that SLC7A11 is downregulated and that p53-mediated ferroptosis is significantly induced in spleens and testis of p533KR/3KRXRCC4-/- mice. These results demonstrate that the direct role of p53-mediated cell cycle arrest, senescence and apoptosis is to control genomic stability in vivo. Our study not only validates the importance of ferroptosis in p53-mediated tumor suppression in vivo but also reveals that the combination of genomic instability and activation of ferroptosis may promote aging-associated phenotypes.
Keywords: acetylation; ferroptosis; genomic instability; p53; tumor suppression.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
Similar articles
-
Interplay of p53 and DNA-repair protein XRCC4 in tumorigenesis, genomic stability and development.Nature. 2000 Apr 20;404(6780):897-900. doi: 10.1038/35009138. Nature. 2000. PMID: 10786799
-
p53 efficiently suppresses tumor development in the complete absence of its cell-cycle inhibitory and proapoptotic effectors p21, Puma, and Noxa.Cell Rep. 2013 May 30;3(5):1339-45. doi: 10.1016/j.celrep.2013.04.012. Epub 2013 May 9. Cell Rep. 2013. PMID: 23665218
-
Inhibition of Mdmx (Mdm4) in vivo induces anti-obesity effects.Oncotarget. 2018 Jan 2;9(7):7282-7297. doi: 10.18632/oncotarget.23837. eCollection 2018 Jan 26. Oncotarget. 2018. PMID: 29484110 Free PMC article.
-
The role of DNA damage responses in p53 biology.Arch Toxicol. 2015 Apr;89(4):501-17. doi: 10.1007/s00204-015-1459-z. Epub 2015 Jan 25. Arch Toxicol. 2015. PMID: 25618545 Review.
-
DNA repair factors and telomere-chromosome integrity in mammalian cells.Cytogenet Genome Res. 2004;104(1-4):116-22. doi: 10.1159/000077475. Cytogenet Genome Res. 2004. PMID: 15162024 Review.
Cited by
-
CLP36 promotes p53 deficient sarcoma progression through suppression of atrophin-1 interacting protein-4 (AIP-4)-dependent degradation of YAP1.Theranostics. 2022 Jul 4;12(11):5051-5068. doi: 10.7150/thno.72365. eCollection 2022. Theranostics. 2022. PMID: 35836803 Free PMC article.
-
Proteomics profiling and pathway analysis of hippocampal aging in rhesus monkeys.BMC Neurosci. 2020 Jan 15;21(1):2. doi: 10.1186/s12868-020-0550-4. BMC Neurosci. 2020. PMID: 31941443 Free PMC article.
-
1,25-D3 attenuates cerebral ischemia injury by regulating mitochondrial metabolism via the AMPK/AKT/GSK3β pathway.Front Aging Neurosci. 2022 Oct 17;14:1015453. doi: 10.3389/fnagi.2022.1015453. eCollection 2022. Front Aging Neurosci. 2022. PMID: 36325190 Free PMC article.
-
Nucleolus-derived mediators in oncogenic stress response and activation of p53-dependent pathways.Histochem Cell Biol. 2016 Aug;146(2):119-39. doi: 10.1007/s00418-016-1443-6. Epub 2016 May 3. Histochem Cell Biol. 2016. PMID: 27142852 Review.
-
p53 and Tumor Suppression: It Takes a Network.Trends Cell Biol. 2021 Apr;31(4):298-310. doi: 10.1016/j.tcb.2020.12.011. Epub 2021 Jan 28. Trends Cell Biol. 2021. PMID: 33518400 Free PMC article. Review.
References
-
- Vousden KH, Prives C. Blinded by the Light: The Growing Complexity of p53. Cell. 2009;137:413–431. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

