The structure and dynamics of secretory component and its interactions with polymeric immunoglobulins
- PMID: 26943617
- PMCID: PMC4786434
- DOI: 10.7554/eLife.10640
The structure and dynamics of secretory component and its interactions with polymeric immunoglobulins
Abstract
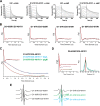
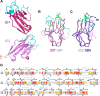
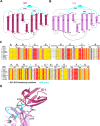
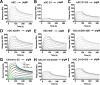
As a first-line vertebrate immune defense, the polymeric immunoglobulin receptor (pIgR) transports polymeric IgA and IgM across epithelia to mucosal secretions, where the cleaved ectodomain (secretory component; SC) becomes a component of secretory antibodies, or when unliganded, binds and excludes bacteria. Here we report the 2.6Å crystal structure of unliganded human SC (hSC) and comparisons with a 1.7Å structure of teleost fish SC (tSC), an early pIgR ancestor. The hSC structure comprises five immunoglobulin-like domains (D1-D5) arranged as a triangle, with an interface between ligand-binding domains D1 and D5. Electron paramagnetic resonance measurements confirmed the D1-D5 interface in solution and revealed that it breaks upon ligand binding. Together with binding studies of mutant and chimeric SCs, which revealed domain contributions to secretory antibody formation, these results provide detailed models for SC structure, address pIgR evolution, and demonstrate that SC uses multiple conformations to protect mammals from pathogens.
Keywords: biophysics; crystal structures; double electron-electron resonance; human; immunology; polymeric ig receptor (pIgR); secretory component (SC); secretory igA/igM; structural biology; surface plasmon resonance.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures


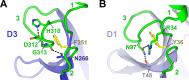
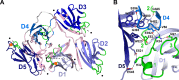

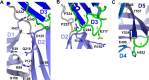
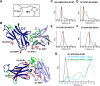


References
-
- Adams PD, Afonine PV, Bunkóczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung L-W, Kapral GJ, Grosse-Kunstleve RW, McCoy AJ, Moriarty NW, Oeffner R, Read RJ, Richardson DC, Richardson JS, Terwilliger TC, Zwart PH. PHENIX : a comprehensive python-based system for macromolecular structure solution. Acta Crystallographica Section D Biological Crystallography. 2010;66:213–221. doi: 10.1107/S0907444909052925. - DOI - PMC - PubMed
-
- Afonine PV, Grosse-Kunstleve RW, Echols N, Headd JJ, Moriarty NW, Mustyakimov M, Terwilliger TC, Urzhumtsev A, Zwart PH, Adams PD. Towards automated crystallographic structure refinement with phenix.refine. Acta Crystallographica Section D Biological Crystallography. 2012;68:352–367. doi: 10.1107/S0907444912001308. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

