Characterization and Localization of Citrullinated Proteoglycan Aggrecan in Human Articular Cartilage
- PMID: 26943656
- PMCID: PMC4778950
- DOI: 10.1371/journal.pone.0150784
Characterization and Localization of Citrullinated Proteoglycan Aggrecan in Human Articular Cartilage
Abstract
Background: Rheumatoid arthritis (RA) is an autoimmune disease of the synovial joints. The autoimmune character of RA is underscored by prominent production of autoantibodies such as those against IgG (rheumatoid factor), and a broad array of joint tissue-specific and other endogenous citrullinated proteins. Anti-citrullinated protein antibodies (ACPA) can be detected in the sera and synovial fluids of RA patients and ACPA seropositivity is one of the diagnostic criteria of RA. Studies have demonstrated that RA T cells respond to citrullinated peptides (epitopes) of proteoglycan (PG) aggrecan, which is one of the most abundant macromolecules of articular cartilage. However, it is not known if the PG molecule is citrullinated in vivo in human cartilage, and if so, whether citrulline-containing neoepitopes of PG (CitPG) can contribute to autoimmunity in RA.
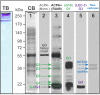
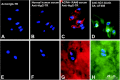
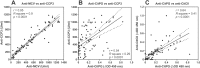
Methods: CitPG was detected in human cartilage extracts using ACPA+ RA sera in dot blot and Western blot. Citrullination status of in vitro citrullinated recombinant G1 domain of human PG (rhG1) was confirmed by antibody-based and chemical methods, and potential sites of citrullination in rhG1 were explored by molecular modeling. CitPG-specific serum autoantibodies were quantified by enzyme-linked immunosorbent assays, and CitPG was localized in osteoarthritic (OA) and RA cartilage using immunohistochemistry.
Findings: Sera from ACPA+ RA patients reacted with PG purified from normal human cartilage specimens. PG fragments (mainly those containing the G1 domain) from OA or RA cartilage extracts were recognized by ACPA+ sera but not by serum from ACPA- individuals. ACPA+ sera also reacted with in vitro citrullinated rhG1 and G3 domain-containing fragment(s) of PG. Molecular modeling suggested multiple sites of potential citrullination within the G1 domain. The immunohistochemical localization of CitPG was different in OA and RA cartilage.
Conclusions: CitPG is a new member of citrullinated proteins identified in human joints. CitPG could be found in both normal and diseased cartilage specimens. Antibodies against CitPG may trigger or augment arthritis by forming immune complexes with this autoantigen in the joints of ACPA+ RA patients.
Conflict of interest statement
Figures



Similar articles
-
Immune Recognition of Citrullinated Proteoglycan Aggrecan Epitopes in Mice with Proteoglycan-Induced Arthritis and in Patients with Rheumatoid Arthritis.PLoS One. 2016 Jul 28;11(7):e0160284. doi: 10.1371/journal.pone.0160284. eCollection 2016. PLoS One. 2016. PMID: 27466816 Free PMC article.
-
Characterization of Autoantigens Targeted by Anti-Citrullinated Protein Antibodies In Vivo: Prominent Role for Epitopes Derived from Histone 4 Proteins.PLoS One. 2016 Oct 27;11(10):e0165501. doi: 10.1371/journal.pone.0165501. eCollection 2016. PLoS One. 2016. PMID: 27788229 Free PMC article.
-
Identification of new citrulline-specific autoantibodies, which bind to human arthritic cartilage, by mass spectrometric analysis of citrullinated type II collagen.Arthritis Rheumatol. 2014 Jun;66(6):1440-9. doi: 10.1002/art.38383. Arthritis Rheumatol. 2014. PMID: 24470447
-
[The pathogenic role of ACPA in rheumatoid arthritis].Nihon Rinsho Meneki Gakkai Kaishi. 2017;40(6):391-395. doi: 10.2177/jsci.40.391. Nihon Rinsho Meneki Gakkai Kaishi. 2017. PMID: 29367523 Review. Japanese.
-
Anti-citrullinated peptides as autoantigens in rheumatoid arthritis-relevance to treatment.Autoimmun Rev. 2014 Nov;13(11):1114-20. doi: 10.1016/j.autrev.2014.08.012. Epub 2014 Aug 23. Autoimmun Rev. 2014. PMID: 25182207 Review.
Cited by
-
Immune Recognition of Citrullinated Proteoglycan Aggrecan Epitopes in Mice with Proteoglycan-Induced Arthritis and in Patients with Rheumatoid Arthritis.PLoS One. 2016 Jul 28;11(7):e0160284. doi: 10.1371/journal.pone.0160284. eCollection 2016. PLoS One. 2016. PMID: 27466816 Free PMC article.
-
Therapeutic Effects of Hypoxic and Pro-Inflammatory Priming of Mesenchymal Stem Cell-Derived Extracellular Vesicles in Inflammatory Arthritis.Int J Mol Sci. 2021 Dec 23;23(1):126. doi: 10.3390/ijms23010126. Int J Mol Sci. 2021. PMID: 35008555 Free PMC article.
-
Mesenchymal Stem Cell-Conditioned Medium Reduces Disease Severity and Immune Responses in Inflammatory Arthritis.Sci Rep. 2017 Dec 21;7(1):18019. doi: 10.1038/s41598-017-18144-w. Sci Rep. 2017. PMID: 29269885 Free PMC article.
-
Extracellular matrix dynamics in vascular remodeling.Am J Physiol Cell Physiol. 2020 Sep 1;319(3):C481-C499. doi: 10.1152/ajpcell.00147.2020. Epub 2020 Jun 24. Am J Physiol Cell Physiol. 2020. PMID: 32579472 Free PMC article. Review.
-
Neutrophil extracellular traps mediate articular cartilage damage and enhance cartilage component immunogenicity in rheumatoid arthritis.JCI Insight. 2020 Jul 9;5(13):e139388. doi: 10.1172/jci.insight.139388. JCI Insight. 2020. PMID: 32484790 Free PMC article.
References
-
- Fox DA. Etiology and Pathogenesis of Rheumatoid Arthritis In: Koopman WJ, Moreland LW, editors. Arthritis and Allied Conditions: A Textbook of Rheumatology. Philadelphia: Lippincott Williams & Wilkins; 2005. pp. 1085–1102.
-
- Firestein GS. Rheumatoid arthritis: Etiology and pathogeneis of rheumatoid arthritis In: Ruddy S, Harris ED, Sledge CB, Kelley WN, editors. Kelley's Textbook of Rheumatology. Philadelphia, PA: W.B.Saunders Co.; 2005. pp. 996–1045.
-
- Bas S, Perneger TV, Seitz M, Tiercy JM, Roux-Lombard P, Guerne PA. Diagnostic tests for rheumatoid arthritis: comparison of anti-cyclic citrullinated peptide antibodies, anti-keratin antibodies and IgM rheumatoid factors. Rheumatology (Oxford) 2002; 41: 809–814 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

