A Numerically Subdominant CD8 T Cell Response to Matrix Protein of Respiratory Syncytial Virus Controls Infection with Limited Immunopathology
- PMID: 26943673
- PMCID: PMC4778879
- DOI: 10.1371/journal.ppat.1005486
A Numerically Subdominant CD8 T Cell Response to Matrix Protein of Respiratory Syncytial Virus Controls Infection with Limited Immunopathology
Abstract
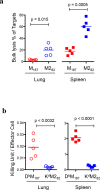
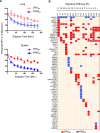
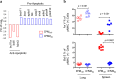
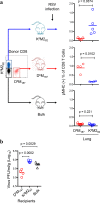
CD8 T cells are involved in pathogen clearance and infection-induced pathology in respiratory syncytial virus (RSV) infection. Studying bulk responses masks the contribution of individual CD8 T cell subsets to protective immunity and immunopathology. In particular, the roles of subdominant responses that are potentially beneficial to the host are rarely appreciated when the focus is on magnitude instead of quality of response. Here, by evaluating CD8 T cell responses in CB6F1 hybrid mice, in which multiple epitopes are recognized, we found that a numerically subdominant CD8 T cell response against DbM187 epitope of the virus matrix protein expressed high avidity TCR and enhanced signaling pathways associated with CD8 T cell effector functions. Each DbM187 T effector cell lysed more infected targets on a per cell basis than the numerically dominant KdM282 T cells, and controlled virus replication more efficiently with less pulmonary inflammation and illness than the previously well-characterized KdM282 T cell response. Our data suggest that the clinical outcome of viral infections is determined by the integrated functional properties of a variety of responding CD8 T cells, and that the highest magnitude response may not necessarily be the best in terms of benefit to the host. Understanding how to induce highly efficient and functional T cells would inform strategies for designing vaccines intended to provide T cell-mediated immunity.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Pulmonary T cells induced by respiratory syncytial virus are functional and can make an important contribution to long-lived protective immunity.Eur J Immunol. 2002 Sep;32(9):2562-9. doi: 10.1002/1521-4141(200209)32:9<2562::AID-IMMU2562>3.0.CO;2-4. Eur J Immunol. 2002. PMID: 12207340
-
Responses against a subdominant CD8+ T cell epitope protect against immunopathology caused by a dominant epitope.J Immunol. 2010 Oct 15;185(8):4673-80. doi: 10.4049/jimmunol.1001606. Epub 2010 Sep 10. J Immunol. 2010. PMID: 20833834 Free PMC article.
-
Efficient lung recruitment of respiratory syncytial virus-specific Th1 cells induced by recombinant bacillus Calmette-Guérin promotes virus clearance and protects from infection.J Immunol. 2010 Dec 15;185(12):7633-45. doi: 10.4049/jimmunol.0903452. Epub 2010 Nov 17. J Immunol. 2010. PMID: 21084664
-
CD8+ T cell immunity against human respiratory syncytial virus.Vaccine. 2014 Oct 21;32(46):6130-7. doi: 10.1016/j.vaccine.2014.08.063. Epub 2014 Sep 16. Vaccine. 2014. PMID: 25223272 Review.
-
Respiratory syncytial virus and T cells: interplay between the virus and the host adaptive immune system.Proc Am Thorac Soc. 2005;2(2):141-6. doi: 10.1513/pats.200503-022AW. Proc Am Thorac Soc. 2005. PMID: 16113482 Review.
Cited by
-
Memory CD8 T cells mediate severe immunopathology following respiratory syncytial virus infection.PLoS Pathog. 2018 Jan 2;14(1):e1006810. doi: 10.1371/journal.ppat.1006810. eCollection 2018 Jan. PLoS Pathog. 2018. PMID: 29293660 Free PMC article.
-
Human respiratory syncytial virus: pathogenesis, immune responses, and current vaccine approaches.Eur J Clin Microbiol Infect Dis. 2018 Oct;37(10):1817-1827. doi: 10.1007/s10096-018-3289-4. Epub 2018 Jun 6. Eur J Clin Microbiol Infect Dis. 2018. PMID: 29876771 Review.
-
Role of CD4+ T Cells in the Control of Viral Infections: Recent Advances and Open Questions.Int J Mol Sci. 2021 Jan 7;22(2):523. doi: 10.3390/ijms22020523. Int J Mol Sci. 2021. PMID: 33430234 Free PMC article. Review.
-
Vaccine development for respiratory syncytial virus.Curr Opin Virol. 2017 Apr;23:107-112. doi: 10.1016/j.coviro.2017.03.012. Epub 2017 May 16. Curr Opin Virol. 2017. PMID: 28525878 Free PMC article. Review.
-
Immunopeptidomic Analysis of BoLA-I and BoLA-DR Presented Peptides from Theileria parva Infected Cells.Vaccines (Basel). 2022 Nov 11;10(11):1907. doi: 10.3390/vaccines10111907. Vaccines (Basel). 2022. PMID: 36423003 Free PMC article.
References
-
- Welliver TP, Garofalo RP, Hosakote Y, Hintz KH, Avendano L, Sanchez K, et al. Severe human lower respiratory tract illness caused by respiratory syncytial virus and influenza virus is characterized by the absence of pulmonary cytotoxic lymphocyte responses. J Infect Dis. 2007;195(8):1126–36. Epub 2007/03/16. doi: JID37165 [pii] 10.1086/512615 . - DOI - PMC - PubMed
-
- El Saleeby CM, Suzich J, Conley ME, DeVincenzo JP. Quantitative effects of palivizumab and donor-derived T cells on chronic respiratory syncytial virus infection, lung disease, and fusion glycoprotein amino acid sequences in a patient before and after bone marrow transplantation. Clin Infect Dis. 2004;39(2):e17–20. Epub 2004/08/13. 10.1086/421779 CID32988 [pii]. . - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

