Towards Improving Point-of-Care Diagnosis of Non-malaria Febrile Illness: A Metabolomics Approach
- PMID: 26943791
- PMCID: PMC4778767
- DOI: 10.1371/journal.pntd.0004480
Towards Improving Point-of-Care Diagnosis of Non-malaria Febrile Illness: A Metabolomics Approach
Abstract
Introduction: Non-malaria febrile illnesses such as bacterial bloodstream infections (BSI) are a leading cause of disease and mortality in the tropics. However, there are no reliable, simple diagnostic tests for identifying BSI or other severe non-malaria febrile illnesses. We hypothesized that different infectious agents responsible for severe febrile illness would impact on the host metabolome in different ways, and investigated the potential of plasma metabolites for diagnosis of non-malaria febrile illness.
Methodology: We conducted a comprehensive mass-spectrometry based metabolomics analysis of the plasma of 61 children with severe febrile illness from a malaria-endemic rural African setting. Metabolite features characteristic for non-malaria febrile illness, BSI, severe anemia and poor clinical outcome were identified by receiver operating curve analysis.
Principal findings: The plasma metabolome profile of malaria and non-malaria patients revealed fundamental differences in host response, including a differential activation of the hypothalamic-pituitary-adrenal axis. A simple corticosteroid signature was a good classifier of severe malaria and non-malaria febrile patients (AUC 0.82, 95% CI: 0.70-0.93). Patients with BSI were characterized by upregulated plasma bile metabolites; a signature of two bile metabolites was estimated to have a sensitivity of 98.1% (95% CI: 80.2-100) and a specificity of 82.9% (95% CI: 54.7-99.9) to detect BSI in children younger than 5 years. This BSI signature demonstrates that host metabolites can have a superior diagnostic sensitivity compared to pathogen-detecting tests to identify infections characterized by low pathogen load such as BSI.
Conclusions: This study demonstrates the potential use of plasma metabolites to identify causality in children with severe febrile illness in malaria-endemic settings.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

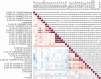

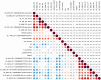


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

