Development and Feasibility Testing of a Critical Care EEG Monitoring Database for Standardized Clinical Reporting and Multicenter Collaborative Research
- PMID: 26943901
- PMCID: PMC4878836
- DOI: 10.1097/WNP.0000000000000230
Development and Feasibility Testing of a Critical Care EEG Monitoring Database for Standardized Clinical Reporting and Multicenter Collaborative Research
Abstract
Purpose: The rapid expansion of the use of continuous critical care electroencephalogram (cEEG) monitoring and resulting multicenter research studies through the Critical Care EEG Monitoring Research Consortium has created the need for a collaborative data sharing mechanism and repository. The authors describe the development of a research database incorporating the American Clinical Neurophysiology Society standardized terminology for critical care EEG monitoring. The database includes flexible report generation tools that allow for daily clinical use.
Methods: Key clinical and research variables were incorporated into a Microsoft Access database. To assess its utility for multicenter research data collection, the authors performed a 21-center feasibility study in which each center entered data from 12 consecutive intensive care unit monitoring patients. To assess its utility as a clinical report generating tool, three large volume centers used it to generate daily clinical critical care EEG reports.
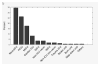
Results: A total of 280 subjects were enrolled in the multicenter feasibility study. The duration of recording (median, 25.5 hours) varied significantly between the centers. The incidence of seizure (17.6%), periodic/rhythmic discharges (35.7%), and interictal epileptiform discharges (11.8%) was similar to previous studies. The database was used as a clinical reporting tool by 3 centers that entered a total of 3,144 unique patients covering 6,665 recording days.
Conclusions: The Critical Care EEG Monitoring Research Consortium database has been successfully developed and implemented with a dual role as a collaborative research platform and a clinical reporting tool. It is now available for public download to be used as a clinical data repository and report generating tool.
Figures


Similar articles
-
Continuous EEG in Pediatric Critical Care: Yield and Efficiency of Seizure Detection.J Clin Neurophysiol. 2017 Sep;34(5):421-426. doi: 10.1097/WNP.0000000000000379. J Clin Neurophysiol. 2017. PMID: 28430674
-
The use and yield of continuous EEG in critically ill patients: A comparative study of three centers.Clin Neurophysiol. 2017 Apr;128(4):570-578. doi: 10.1016/j.clinph.2017.01.001. Epub 2017 Jan 17. Clin Neurophysiol. 2017. PMID: 28231475
-
American clinical neurophysiology society standardized EEG terminology and categorization for the description of continuous EEG monitoring in neonates: report of the American Clinical Neurophysiology Society critical care monitoring committee.J Clin Neurophysiol. 2013 Apr;30(2):161-73. doi: 10.1097/WNP.0b013e3182872b24. J Clin Neurophysiol. 2013. PMID: 23545767 Review. No abstract available.
-
Baseline EEG pattern on continuous ICU EEG monitoring and incidence of seizures.J Clin Neurophysiol. 2015 Apr;32(2):147-51. doi: 10.1097/WNP.0000000000000157. J Clin Neurophysiol. 2015. PMID: 25437330
-
The standardization debate: A conflation trap in critical care electroencephalography.Seizure. 2015 Jan;24:52-8. doi: 10.1016/j.seizure.2014.09.017. Epub 2014 Oct 16. Seizure. 2015. PMID: 25457454 Free PMC article. Review.
Cited by
-
Continuous Electroencephalography After Moderate to Severe Traumatic Brain Injury.Crit Care Med. 2019 Apr;47(4):574-582. doi: 10.1097/CCM.0000000000003639. Crit Care Med. 2019. PMID: 30624278 Free PMC article.
-
Developing an Electroencephalogram-based Model to Predict Awakening after Cardiac Arrest Using Partial Processing with the BIS Engine.Anesthesiology. 2025 May 1;142(5):806-817. doi: 10.1097/ALN.0000000000005369. Epub 2025 Jan 9. Anesthesiology. 2025. PMID: 39786948
-
Glioma genetic profiles associated with electrophysiologic hyperexcitability.medRxiv [Preprint]. 2023 Feb 24:2023.02.22.23285841. doi: 10.1101/2023.02.22.23285841. medRxiv. 2023. Update in: Neuro Oncol. 2024 Feb 2;26(2):323-334. doi: 10.1093/neuonc/noad176. PMID: 36865325 Free PMC article. Updated. Preprint.
-
Early EEG hyperexcitability is associated with decreased survival in newly diagnosed IDH-wildtype glioma.J Neurooncol. 2022 Aug;159(1):211-218. doi: 10.1007/s11060-022-04059-8. Epub 2022 Jun 17. J Neurooncol. 2022. PMID: 35715666 Free PMC article.
-
The role of cEEG as a predictor of patient outcome and survival in patients with intraparenchymal hemorrhages.Seizure. 2018 Oct;61:122-127. doi: 10.1016/j.seizure.2018.08.014. Epub 2018 Aug 15. Seizure. 2018. PMID: 30138824 Free PMC article.
References
-
- Aurlien H, Gjerde IO, Gilhus NE, et al. A new way of building a database of EEG findings. Clin Neurophysiol. 1999;110:986–995. - PubMed
-
- Beniczky S, Hirsch LJ, Kaplan PW, et al. Unified EEG terminology and criteria for nonconvulsive status epilepticus. Epilepsia. 2013b;54(suppl 6):28–29. - PubMed
-
- Chong DJ, Hirsch LJ. Which EEG patterns warrant treatment in the critically ill? reviewing the evidence for treatment of periodic epileptiform discharges and related patterns. J Clin Neurophysiol. 2005;22:79–91. - PubMed
-
- Claassen J, Mayer SA, Kowalski RG, et al. Detection of electrographic seizures with continuous EEG monitoring in critically ill patients. Neurology. 2004;62:1743–1748. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

