The Vaccine Adjuvant Chitosan Promotes Cellular Immunity via DNA Sensor cGAS-STING-Dependent Induction of Type I Interferons
- PMID: 26944200
- PMCID: PMC4852885
- DOI: 10.1016/j.immuni.2016.02.004
The Vaccine Adjuvant Chitosan Promotes Cellular Immunity via DNA Sensor cGAS-STING-Dependent Induction of Type I Interferons
Abstract
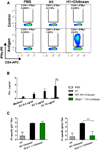
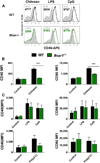
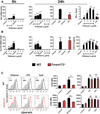
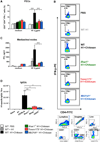
The cationic polysaccharide chitosan is an attractive candidate adjuvant capable of driving potent cell-mediated immunity, but the mechanism by which it acts is not clear. We show that chitosan promotes dendritic cell maturation by inducing type I interferons (IFNs) and enhances antigen-specific T helper 1 (Th1) responses in a type I IFN receptor-dependent manner. The induction of type I IFNs, IFN-stimulated genes and dendritic cell maturation by chitosan required the cytoplasmic DNA sensor cGAS and STING, implicating this pathway in dendritic cell activation. Additionally, this process was dependent on mitochondrial reactive oxygen species and the presence of cytoplasmic DNA. Chitosan-mediated enhancement of antigen specific Th1 and immunoglobulin G2c responses following vaccination was dependent on both cGAS and STING. These findings demonstrate that a cationic polymer can engage the STING-cGAS pathway to trigger innate and adaptive immune responses.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures


Comment in
-
Chitosan: An Adjuvant with an Unanticipated STING.Immunity. 2016 Mar 15;44(3):522-524. doi: 10.1016/j.immuni.2016.03.002. Immunity. 2016. PMID: 26982361
References
-
- Bossie A, Vitetta ES. IFN-γ enhances secretion of IgG2a from IgG2a-committed LPS-stimulated murine B cells: implications for the role of IFN-γ in class switching. Cell. Immunol. 1991;135:95–104. - PubMed
-
- Brewer JM, Conacher M, Hunter CA, Mohrs M, Brombacher F, Alexander J. Aluminium hydroxide adjuvant initiates strong antigen-specific Th2 responses in the absence of IL-4- or IL-13-mediated signaling. J. Immunol. 1999;163:6448–6454. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

