Novel Entropically Driven Conformation-specific Interactions with Tomm34 Protein Modulate Hsp70 Protein Folding and ATPase Activities
- PMID: 26944342
- PMCID: PMC4858950
- DOI: 10.1074/mcp.M116.058131
Novel Entropically Driven Conformation-specific Interactions with Tomm34 Protein Modulate Hsp70 Protein Folding and ATPase Activities
Abstract
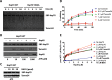
Co-chaperones containing tetratricopeptide repeat (TPR) domains enable cooperation between Hsp70 and Hsp90 to maintain cellular proteostasis. Although the details of the molecular interactions between some TPR domains and heat shock proteins are known, we describe a novel mechanism by which Tomm34 interacts with and coordinates Hsp70 activities. In contrast to the previously defined Hsp70/Hsp90-organizing protein (Hop), Tomm34 interaction is dependent on the Hsp70 chaperone cycle. Tomm34 binds Hsp70 in a complex process; anchorage of the Hsp70 C terminus by the TPR1 domain is accompanied by additional contacts formed exclusively in the ATP-bound state of Hsp70 resulting in a high affinity entropically driven interaction. Tomm34 induces structural changes in determinants within the Hsp70-lid subdomain and modulates Hsp70/Hsp40-mediated refolding and Hsp40-stimulated Hsp70 ATPase activity. Because Tomm34 recruits Hsp90 through its TPR2 domain, we propose a model in which Tomm34 enables Hsp70/Hsp90 scaffolding and influences the Hsp70 chaperone cycle, providing an additional role for co-chaperones that contain multiple TPR domains in regulating protein homeostasis.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures









References
-
- Young J. C., Agashe V. R., Siegers K., and Hartl F. U. (2004) Pathways of chaperone-mediated protein folding in the cytosol. Nat. Rev. Mol. Cell Biol. 5, 781–791 - PubMed
-
- Mayer M. P. (2010) Gymnastics of molecular chaperones. Mol. Cell 39, 321–331 - PubMed
-
- Alvira S., Cuéllar J., Röhl A., Yamamoto S., Itoh H., Alfonso C., Rivas G., Buchner J., and Valpuesta J. M. (2014) Structural characterization of the substrate transfer mechanism in Hsp70/Hsp90 folding machinery mediated by Hop. Nat. Commun. 5, 5484. - PubMed
-
- Zhang H., Amick J., Chakravarti R., Santarriaga S., Schlanger S., McGlone C., Dare M., Nix J. C., Scaglione K. M., Stuehr D. J., Misra S., and Page R. C. (2015) A bipartite interaction between Hsp70 and CHIP regulates ubiquitination of chaperoned client proteins. Structure 23, 472–482 - PMC - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

