Using a tailored health information technology- driven intervention to improve health literacy and medication adherence in a Pakistani population with vascular disease (Talking Rx) - study protocol for a randomized controlled trial
- PMID: 26944938
- PMCID: PMC4779210
- DOI: 10.1186/s13063-016-1244-1
Using a tailored health information technology- driven intervention to improve health literacy and medication adherence in a Pakistani population with vascular disease (Talking Rx) - study protocol for a randomized controlled trial
Abstract
Background: Vascular disease, manifesting as myocardial infarction and stroke, is a major cause of morbidity and mortality, especially in low- and middle-income countries. Current estimates are that only one in six patients have good adherence to medications and very few have sufficient health literacy. Our aim is to explore the effectiveness and acceptability of Prescription Interactive Voice Response (IVR) Talking Prescriptions (Talking Rx) and SMS reminders in increasing medication adherence and health literacy in Pakistani patients with vascular disease.
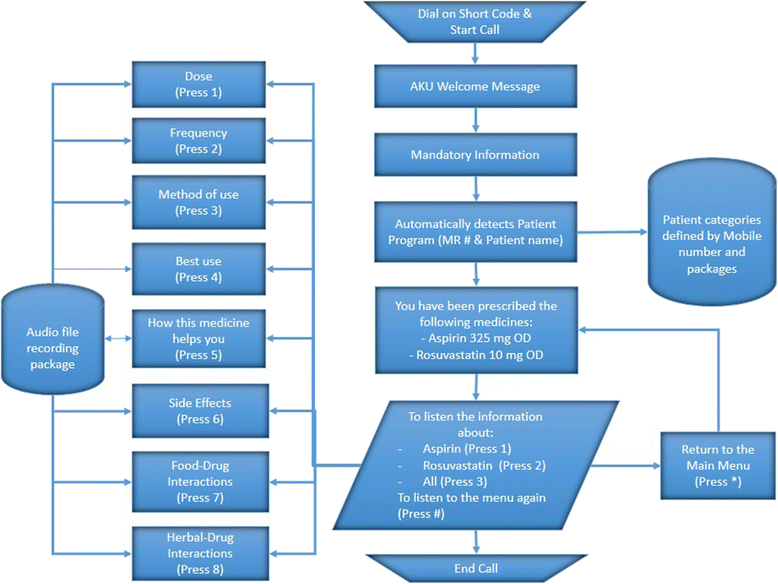
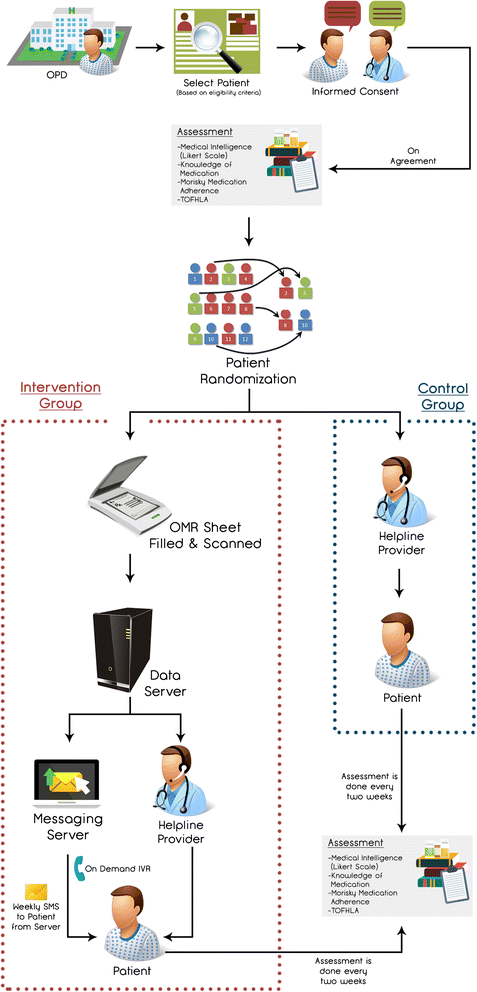
Methods: This is a randomized, controlled, single center trial. Adult participants, with access to a cell phone and a history of vascular disease, taking multiple risk-modifying medications (inclusive of anti-platelets and statins) will be selected from cerebrovascular and cardiovascular clinics. They will be randomized in a 1:1 ratio via a block design to the intervention or the control arm with both groups having access to a helpline number to address their queries in addition to standard of care as per institutional guidelines. Participants in the intervention group will also have access to Interactive Voice Response (IVR) technology tailored to their respective prescriptions in the native language (Urdu) and will have the ability to hear information about their medication dosage, correct use, side effects, mechanism of action and how and why they should use their medication, as many times as they like. Participants in the intervention arm will also receive scheduled SMS messages reminding them to take their medications. The primary outcome measure will be the comparison of the difference in adherence to anti-platelet and statin medication between baseline and at 3-month follow-up in each group measured by the Morisky Medication Adherence Scale. To ascertain the impact of our intervention on health literacy, we will also compare a local content-validated and modified version of Test of Health Literacy in Adults (TOFHLA) between the intervention and the control arm. We estimate that a sample size of 86 participants in each arm will be able to detect a difference of 1 point on the MMAS with a power of 90 % and significance level of 5 %. Accounting for an attrition rate of 15 %, we plan to enroll 100 participants in each arm (total study population = 200). We hypothesize that a linguistically tailored health IT intervention based on IVR and SMS will be associated with an improvement in adherence (to anti-platelet and lipid-lowering medications) and an improvement in health literacy in Pakistani patients with vascular disease.
Discussion: This innovative study will provide early data for the feasibility of the use of IT based prescriptions in an lower middle incorme country setting with limited numeracy and literacy skills.
Trial registration: Clinical Trials.gov: NCT02354040 - 2 February 2015.
Figures

References
-
- Bandura A. Self-efficacy: the exercise of control. New York: Freeman; 1997.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

