Structural Insights into the Transport Mechanism of the Human Sodium-dependent Lysophosphatidylcholine Transporter MFSD2A
- PMID: 26945070
- PMCID: PMC4850279
- DOI: 10.1074/jbc.M116.721035
Structural Insights into the Transport Mechanism of the Human Sodium-dependent Lysophosphatidylcholine Transporter MFSD2A
Abstract
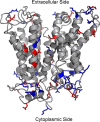
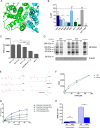
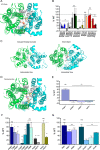
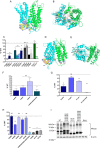
Major facilitator superfamily domain containing 2A (MFSD2A) was recently characterized as a sodium-dependent lysophosphatidylcholine transporter expressed at the blood-brain barrier endothelium. It is the primary route for importation of docosohexaenoic acid and other long-chain fatty acids into fetal and adult brain and is essential for mouse and human brain growth and function. Remarkably, MFSD2A is the first identified major facilitator superfamily member that uniquely transports lipids, implying that MFSD2A harbors unique structural features and transport mechanism. Here, we present three three-dimensional structural models of human MFSD2A derived by homology modeling using MelB- and LacY-based crystal structures and refined by biochemical analysis. All models revealed 12 transmembrane helices and connecting loops and represented the partially outward-open, outward-partially occluded, and inward-open states of the transport cycle. In addition to a conserved sodium-binding site, three unique structural features were identified as follows: a phosphate headgroup binding site, a hydrophobic cleft to accommodate a hydrophobic hydrocarbon tail, and three sets of ionic locks that stabilize the outward-open conformation. Ligand docking studies and biochemical assays identified Lys-436 as a key residue for transport. It is seen forming a salt bridge with the negative charge on the phosphate headgroup. Importantly, MFSD2A transported structurally related acylcarnitines but not a lysolipid without a negative charge, demonstrating the necessity of a negatively charged headgroup interaction with Lys-436 for transport. These findings support a novel transport mechanism by which lysophosphatidylcholines are "flipped" within the transporter cavity by pivoting about Lys-436 leading to net transport from the outer to the inner leaflet of the plasma membrane.
Keywords: X-ray crystallography; blood-brain barrier; brain metabolism; docosahexaenoic acid; drug transport; lysophospholipid; membrane protein; membrane transporter; microcephaly; polyunsaturated fatty acid (PUFA).
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


References
-
- Salem N. Jr., Litman B., Kim H. Y., and Gawrisch K. (2001) Mechanisms of action of docosahexaenoic acid in the nervous system. Lipids 36, 945–959 - PubMed
-
- Edmond J., Higa T. A., Korsak R. A., Bergner E. A., and Lee W. N. (1998) Fatty acid transport and utilization for the developing brain. J. Neurochem. 70, 1227–1234 - PubMed
-
- Lagarde M., Bernoud N., Brossard N., Lemaitre-Delaunay D., Thiès F., Croset M., and Lecerf J. (2001) Lysophosphatidylcholine as a preferred carrier form of docosahexaenoic acid to the brain. J. Mol. Neurosci. 16, 201–204 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

