Coiled-coil domain containing 42 (Ccdc42) is necessary for proper sperm development and male fertility in the mouse
- PMID: 26945718
- PMCID: PMC4826836
- DOI: 10.1016/j.ydbio.2016.01.042
Coiled-coil domain containing 42 (Ccdc42) is necessary for proper sperm development and male fertility in the mouse
Abstract
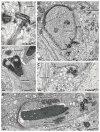
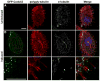
Spermiogenesis is the differentiation of spermatids into motile sperm consisting of a head and a tail. The head harbors a condensed elongated nucleus partially covered by the acrosome-acroplaxome complex. Defects in the acrosome-acroplaxome complex are associated with abnormalities in sperm head shaping. The head-tail coupling apparatus (HTCA), a complex structure consisting of two cylindrical microtubule-based centrioles and associated components, connects the tail or flagellum to the sperm head. Defects in the development of the HTCA cause sperm decapitation and disrupt sperm motility, two major contributors to male infertility. Here, we provide data indicating that mutations in the gene Coiled-coil domain containing 42 (Ccdc42) is associated with malformation of the mouse sperm flagella. In contrast to many other flagella and motile cilia genes, Ccdc42 expression is only observed in the brain and developing sperm. Male mice homozygous for a loss-of-function Ccdc42 allele (Ccdc42(KO)) display defects in the number and location of the HTCA, lack flagellated sperm, and are sterile. The testes enriched expression of Ccdc42 and lack of other phenotypes in mutant mice make it an ideal candidate for screening cases of azoospermia in humans.
Keywords: Ccdc42; Infertility; Intramanchette transport; Sperm; Sperm head–tail coupling apparatus.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures



References
-
- Blanchon S, Legendre M, Copin B, Duquesnoy P, Montantin G, Kott E, Dastot F, Jeanson L, Cachanado M, Rousseau A, Papon JF, Beydon N, Brouard J, Crestani B, Deschildre A, Desir J, Dollfus H, Leheup B, Tamalet A, Thumerelle C, Vojtek AM, Escalier D, Coste A, de Blic J, Clement A, Escudier E, Amselem S. Delineation of CCDC39/CCDC40 mutation spectrum and associated phenotypes in primary ciliary dyskinesia. J Med Genet. 2012;49:410–416. - PubMed
-
- Chizhikov VV, Davenport J, Zhang Q, Shih EK, Cabello OA, Fuchs JL, Yoder BK, Millen KJ. Cilia proteins control cerebellar morphogenesis by promoting expansion of the granule progenitor pool. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:9780–9789. - PMC - PubMed
-
- De Kretser DM, Baker HW. Infertility in men: recent advances and continuing controversies. The Journal of clinical endocrinology and metabolism. 1999;84:3443–3450. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

