Moderate and Severe Inflammatory Acne Vulgaris Effectively Treated with Single-Agent Therapy by a New Fixed-Dose Combination Adapalene 0.3 %/Benzoyl Peroxide 2.5 % Gel: A Randomized, Double-Blind, Parallel-Group, Controlled Study
- PMID: 26945741
- PMCID: PMC4863916
- DOI: 10.1007/s40257-016-0178-4
Moderate and Severe Inflammatory Acne Vulgaris Effectively Treated with Single-Agent Therapy by a New Fixed-Dose Combination Adapalene 0.3 %/Benzoyl Peroxide 2.5 % Gel: A Randomized, Double-Blind, Parallel-Group, Controlled Study
Abstract
Background: A need exists for topical treatments in managing more severe inflammatory acne.
Objectives: The objectives of this study were to evaluate the efficacy and safety of adapalene 0.3 %/benzoyl peroxide 2.5 % (0.3 % A/BPO) topical gel in subjects with moderate and severe inflammatory acne.
Methods: This was a multicenter, randomized, double-blind, parallel-group study. Randomization was stratified by acne severity (50 % moderate and 50 % severe). Subjects received 0.3 % A/BPO, 0.1 % A/BPO (benchmark), or vehicle (comparator) once daily for 12 weeks. Co-primary efficacy endpoints were success rate at week 12 (the percentage of subjects rated 'clear' or 'almost clear' with at least a 2-grade improvement on Investigator's Global Assessment [IGA]) and change in inflammatory (IN) and noninflammatory (NIN) lesion counts from baseline to week 12. Secondary efficacy endpoints were percent changes in IN and NIN lesion counts. Safety endpoints were incidence of adverse events (AEs) and local tolerability signs/symptoms.
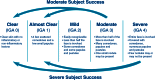
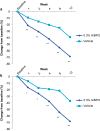
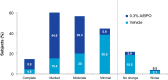
Results: A total of 503 subjects were randomized: 217, 217, and 69 subjects in the 0.3 % A/BPO, 0.1 % A/BPO, and vehicle groups, respectively. For success rate (subjects rated 'clear' or 'almost clear' with ≥2-grade improvement in IGA), 0.3 % A/BPO was superior to vehicle, with a treatment difference of 22.7 % (33.7 vs. 11.0 %; 95 % confidence interval [CI] 12.8-32.6, p < 0.001). At week 12, 0.3 % A/BPO was superior to vehicle for mean reduction from baseline in IN (27.0 vs. 14.4) and NIN lesion counts (40.2 vs. 18.5), as well as for percentage reduction from baseline in IN (68.7 vs. 39.2 %) and NIN lesion counts (68.3 vs. 37.4 %) (all p < 0.001). Among subjects with severe inflammatory acne (IGA = 4), 0.1 % A/BPO did not reach statistical significance for success rate compared with vehicle (p = 0.443), whereas 0.3 % A/BPO demonstrated significantly greater efficacy (p = 0.029, requiring ≥3-point IGA improvement). Additionally, 0.3 % A/BPO was safe and well-tolerated.
Conclusions: Results of this clinical trial demonstrate the significantly greater efficacy of adapalene 0.3 % A/BPO topical gel compared with vehicle as well as a good safety profile in the treatment of moderate to severe inflammatory non-nodulocystic acne, which increases patients' treatment options. CLINICALTRIALS.
Gov identifier: NCT01880320.
Figures



Similar articles
-
Customized Single-agent Therapy Management of Severe Inflammatory Acne: A Randomized, Double-blind, Parallel-group, Controlled Study of a New Treatment--Adapalene 0.3%-Benzoyl Peroxide 2.5% Gel.J Drugs Dermatol. 2015 Dec;14(12):1427-35. J Drugs Dermatol. 2015. PMID: 26659936 Clinical Trial.
-
Preadolescent moderate acne vulgaris: a randomized trial of the efficacy and safety of topical adapalene-benzoyl peroxides.J Drugs Dermatol. 2013 Jun 1;12(6):611-8. J Drugs Dermatol. 2013. PMID: 23839175 Clinical Trial.
-
A 6-month maintenance therapy with adapalene-benzoyl peroxide gel prevents relapse and continuously improves efficacy among patients with severe acne vulgaris: results of a randomized controlled trial.Br J Dermatol. 2011 Jun;164(6):1376-82. doi: 10.1111/j.1365-2133.2011.10344.x. Epub 2011 May 20. Br J Dermatol. 2011. PMID: 21457209 Clinical Trial.
-
Adapalene 0.1%/benzoyl peroxide 2.5% gel: a review of its use in the treatment of acne vulgaris in patients aged ≥ 12 years.Am J Clin Dermatol. 2011 Dec 1;12(6):407-20. doi: 10.2165/11208170-000000000-00000. Am J Clin Dermatol. 2011. PMID: 21967116 Review.
-
Adapalene 0.1% and benzoyl peroxide 2.5%: a novel combination for treatment of acne vulgaris.Skin Therapy Lett. 2009 Jul-Aug;14(6):4-5. Skin Therapy Lett. 2009. PMID: 19609474 Review.
Cited by
-
Investigating the Use of 0.3% Adapalene/2.5% Benzoyl Peroxide Gel for the Management of Moderate-to-Severe Acne in Indian Patients: A Phase 4 Study Assessing Safety and Efficacy.Cureus. 2024 Jul 31;16(7):e65894. doi: 10.7759/cureus.65894. eCollection 2024 Jul. Cureus. 2024. PMID: 39219919 Free PMC article.
-
Why Topical Retinoids Are Mainstay of Therapy for Acne.Dermatol Ther (Heidelb). 2017 Sep;7(3):293-304. doi: 10.1007/s13555-017-0185-2. Epub 2017 Jun 5. Dermatol Ther (Heidelb). 2017. PMID: 28585191 Free PMC article. Review.
-
BPX-01 Minocycline Topical Gel Shows Promise for the Treatment of Moderate-to-severe Inflammatory Acne Vulgaris.J Clin Aesthet Dermatol. 2018 Nov;11(11):25-35. Epub 2018 Nov 1. J Clin Aesthet Dermatol. 2018. PMID: 30588271 Free PMC article.
-
Automated Facial Acne Lesion Detecting and Counting Algorithm for Acne Severity Evaluation and Its Utility in Assisting Dermatologists.Am J Clin Dermatol. 2023 Jul;24(4):649-659. doi: 10.1007/s40257-023-00777-5. Epub 2023 May 9. Am J Clin Dermatol. 2023. PMID: 37160644
-
Synchronizing Pharmacotherapy in Acne with Review of Clinical Care.Indian J Dermatol. 2017 Jul-Aug;62(4):341-357. doi: 10.4103/ijd.IJD_41_17. Indian J Dermatol. 2017. PMID: 28794543 Free PMC article. Review.
References
-
- Pariser DM, Thiboutot DM, Clark SD, et al. The efficacy and safety of adapalene gel 0.3% in the treatment of acne vulgaris: a randomized, multicenter, investigator-blinded, controlled comparison study versus adapalene gel 0.1% and vehicle. Cutis. 2005;76(2):145–151. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

