Destruction of Opportunistic Pathogens via Polymer Nanoparticle-Mediated Release of Plant-Based Antimicrobial Payloads
- PMID: 26946055
- PMCID: PMC5474087
- DOI: 10.1002/adhm.201500974
Destruction of Opportunistic Pathogens via Polymer Nanoparticle-Mediated Release of Plant-Based Antimicrobial Payloads
Abstract
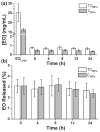
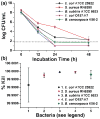
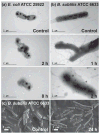
The synthesis of antimicrobial thymol/carvacrol-loaded polythioether nanoparticles (NPs) via a one-pot, solvent-free miniemulsion thiol-ene photopolymerization process is reported. The active antimicrobial agents, thymol and carvacrol, are employed as "solvents" for the thiol-ene monomer phase in the miniemulsion to enable facile high capacity loading (≈50% w/w), excellent encapsulation efficiencies (>95%), and elimination of all postpolymerization purification processes. The NPs serve as high capacity reservoirs for slow-release and delivery of thymol/carvacrol-combination payloads that exhibit inhibitory and bactericidal activity (>99.9% kill efficiency at 24 h) against gram-positive and gram-negative bacteria, including both saprophytic (Bacillus subtilis ATCC 6633 and Escherichia coli ATCC 25922) and pathogenic species (E. coli ATCC 43895, Staphylococcus aureus RN6390, and Burkholderia cenocepacia K56-2). This report is among the first to demonstrate antimicrobial efficacy of essential oil-loaded nanoparticles against B. cenocepacia - an innately resistant opportunistic pathogen commonly associated with debilitating respiratory infections in cystic fibrosis. Although a model platform, these results point to promising pathways to particle-based delivery of plant-derived extracts for a range of antimicrobial applications, including active packaging materials, topical antiseptics, and innovative therapeutics.
Keywords: antimicrobial; carvacrol; miniemulsion polymerization; polymer nanoparticles; thymol.
© 2016 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures




References
-
- [accessed:November 2015]; www.cdc.gov/drugresistance/threat-report-2013/
-
- Mahenthiralingam E, Baldwin A, Dowson CG. J Appl Microbiol. 2008;104:1539. - PubMed
-
- Holden M, Seth-Smith H, Crossman L, Sebaihia M, Bentley S, Cerdeño-Tárraga A, Thomson N, Bason N, Quail M, Sharp S, Cherevach I, Churcher C, Goodhead I, Hauser H, Holroyd N, Mungall K, Scott P, Walker D, White B, Rose H, Iversen P, Mil-Homens D, Rocha E, Fialho A, Baldwin A, Dowson C, Barrell B, Govan J, Vandamme P, Hart CA, Mahenthiralingam E, Parkhill J. J Bacteriol. 2009;191:261. - PMC - PubMed
-
- Drevinek P, Mahenthiralingam E. Clin Microbiol Infect. 2010;16:821. - PubMed
-
- Majeed H, Bian YY, Ali B, Jamil A, Majeed U, Khan QF, Iqbal KJ, Shoemaker CF, Fang Z. RSC Adv. 2015;5:58449.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

