Comparison of PCSK9 Inhibitor Evolocumab vs Ezetimibe in Statin-Intolerant Patients: Design of the Goal Achievement After Utilizing an Anti-PCSK9 Antibody in Statin-Intolerant Subjects 3 (GAUSS-3) Trial
- PMID: 26946077
- PMCID: PMC6490723
- DOI: 10.1002/clc.22518
Comparison of PCSK9 Inhibitor Evolocumab vs Ezetimibe in Statin-Intolerant Patients: Design of the Goal Achievement After Utilizing an Anti-PCSK9 Antibody in Statin-Intolerant Subjects 3 (GAUSS-3) Trial
Abstract
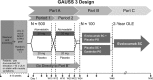
Statins are the accepted standard for lowering low-density lipoprotein cholesterol (LDL-C). However, 5% to 10% of statin-treated patients report intolerance, mostly due to muscle-related adverse effects. Challenges exist to objective identification of statin-intolerant patients. Evolocumab is a monoclonal antibody that binds proprotein convertase subtilisin/kexin type 9 (PCSK9), resulting in marked LDL-C reduction. We report the design of Goal Achievement After Utilizing an Anti-PCSK9 Antibody in Statin-Intolerant Subjects 3 (GAUSS-3), a phase 3, multicenter, randomized, double-blind, ezetimibe-controlled study to compare effectiveness of 24 weeks of evolocumab 420 mg monthly vs ezetimibe 10 mg daily in hypercholesterolemic patients unable to tolerate an effective statin dose. The study incorporates a novel atorvastatin-controlled, double-blind, crossover phase to objectively identify statin intolerance. Eligible patients had LDL-C above the National Cholesterol Education Project Adult Treatment Panel III target level for the appropriate coronary heart disease risk category and were unable to tolerate ≥3 statins or 2 statins (one of which was atorvastatin ≤10 mg/d) or had a history of marked creatine kinase elevation accompanied by muscle symptoms while on 1 statin. This trial has 2 co-primary endpoints: mean percent change from baseline in LDL-C at weeks 22 and 24 and percent change from baseline in LDL-C at week 24. Key secondary efficacy endpoints include change from baseline in LDL-C, percent of patients attaining LDL-C <70 mg/dL (1.81 mmol/L), and percent change from baseline in total cholesterol, non-high-density lipoprotein cholesterol, and apolipoprotein B. Recruitment of 511 patients was completed on November 28, 2014.
© 2016 Wiley Periodicals, Inc.
Figures
References
-
- Bruckert E, Hayem G, Dejager S, et al. Mild to moderate muscular symptoms with high‐dosage statin therapy in hyperlipidemic patients—the PRIMO study. Cardiovasc Drugs Ther. 2005;19:403–414. - PubMed
-
- Thompson PD, Clarkson P, Karas RH. Statin‐associated myopathy. JAMA. 2003;289:1681–1690. - PubMed
-
- Rosenson RS, Baker SK, Jacobson TA, Kopecky SL, Parker BA. An assessment by the Statin Muscle Safety Task Force: 2014 update. J Clin Lipidol. 2014;8:S58–71. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

