In vivo imaging reveals rapid astrocyte depletion and axon damage in a model of neuromyelitis optica-related pathology
- PMID: 26946517
- PMCID: PMC5021140
- DOI: 10.1002/ana.24630
In vivo imaging reveals rapid astrocyte depletion and axon damage in a model of neuromyelitis optica-related pathology
Abstract
Objective: Neuromyelitis optica (NMO) is an autoimmune disease of the central nervous system, which resembles multiple sclerosis (MS). NMO differs from MS, however, in the distribution and histology of neuroinflammatory lesions and shows a more aggressive clinical course. Moreover, the majority of NMO patients carry immunoglobulin G autoantibodies against aquaporin-4 (AQP4), an astrocytic water channel. Antibodies against AQP4 can damage astrocytes by complement, but NMO histopathology also shows demyelination, and - importantly-axon injury, which may determine permanent deficits following NMO relapses. The dynamics of astrocyte injury in NMO and the mechanisms by which toxicity spreads to axons are not understood.
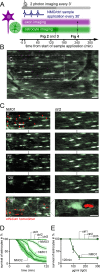
Methods: Here, we establish in vivo imaging of the spinal cord, one of the main sites of NMO pathology, as a powerful tool to study the formation of experimental NMO-related lesions caused by human AQP4 antibodies in mice.
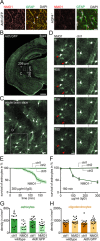
Results: We found that human AQP4 antibodies caused acute astrocyte depletion with initial oligodendrocyte survival. Within 2 hours of antibody application, we observed secondary axon injury in the form of progressive swellings. Astrocyte toxicity and axon damage were dependent on AQP4 antibody titer and complement, specifically C1q.
Interpretation: In vivo imaging of the spinal cord reveals the swift development of NMO-related acute axon injury after AQP4 antibody-mediated astrocyte depletion. This approach will be useful in studying the mechanisms underlying the spread of NMO pathology beyond astrocytes, as well as in evaluating potential neuroprotective interventions. Ann Neurol 2016;79:794-805.
© 2016 The Authors. Annals of Neurology published by Wiley Periodicals, Inc. on behalf of American Neurological Association.
Figures


References
-
- Coleman MP, Perry VH. Axon pathology in neurological disease: a neglected therapeutic target. Trends Neurosci 2002;25:532–537. - PubMed
-
- Trapp BD, Peterson J, Ransohoff RM, et al. Axonal transection in the lesions of multiple sclerosis. N Engl J Med 1998;338:278–285. - PubMed
-
- Wingerchuk DM, Lennon VA, Lucchinetti CF, et al. The spectrum of neuromyelitis optica. Lancet Neurol 2007;6:805–815. - PubMed
-
- Lennon VA, Wingerchuk DM, Kryzer TJ, et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet 2004;364:2106–2112. - PubMed
Grants and funding
- EY022936/NH/NIH HHS/United States
- Guthy-Jackson Foundation
- DFG Priority Program 1710
- 616791/European Research Council under the European Union's Seventh Framework Program FP/2007-2013
- Swiss MS Society
- German Center for Neurodegenerative Disease (DZNE)
- DFG Collaborative Research Center 870
- EXC 1010/Munich Center for Systems Neurology SyNergy
- Klinikum rechts der Isar of the Technical University of Munich
- Transregio 128/Deutsche Forschungsgemeinschaft DFG
- Gebert-Rüf Foundation
- R01 EY022936/EY/NEI NIH HHS/United States
- 616791/ERC_/European Research Council/International
- PP00P3_152928/SNSF_/Swiss National Science Foundation/Switzerland
- UM1 AI110503/AI/NIAID NIH HHS/United States
- Klaus-Tschira Foundation
- Munich, EXC 114/DFG Center for Integrated Protein Science
- German Federal Ministry of Research and Education (BMBF; Competence Network Multiple Sclerosis)
- National MS Society
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

