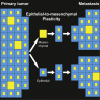
Plasticity between Epithelial and Mesenchymal States Unlinks EMT from Metastasis-Enhancing Stem Cell Capacity
- PMID: 26947068
- PMCID: PMC4802222
- DOI: 10.1016/j.celrep.2016.02.034
Plasticity between Epithelial and Mesenchymal States Unlinks EMT from Metastasis-Enhancing Stem Cell Capacity
Abstract
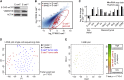
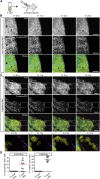
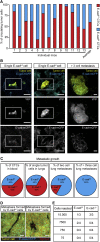
Forced overexpression and/or downregulation of proteins regulating epithelial-to-mesenchymal transition (EMT) has been reported to alter metastasis by changing migration and stem cell capacity of tumor cells. However, these manipulations artificially keep cells in fixed states, while in vivo cells may adapt transient and reversible states. Here, we have tested the existence and role of epithelial-mesenchymal plasticity in metastasis of mammary tumors without artificially modifying EMT regulators. In these tumors, we found by intravital microscopy that the motile tumor cells have undergone EMT, while their epithelial counterparts were not migratory. Moreover, we found that epithelial-mesenchymal plasticity renders any EMT-induced stemness differences, as reported previously, irrelevant for metastatic outgrowth, because mesenchymal cells that arrive at secondary sites convert to the epithelial state within one or two divisions, thereby obtaining the same stem cell potential as their arrived epithelial counterparts. We conclude that epithelial-mesenchymal plasticity supports migration but additionally eliminates stemness-enhanced metastatic outgrowth differences.
Keywords: cancer; epithelial-to-mesenchymal transition (EMT); intravital microscopy.
Copyright © 2016 The Authors. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Beck B., Lapouge G., Rorive S., Drogat B., Desaedelaere K., Delafaille S., Dubois C., Salmon I., Willekens K., Marine J.C., Blanpain C. Different levels of Twist1 regulate skin tumor initiation, stemness, and progression. Cell Stem Cell. 2015;16:67–79. - PubMed
-
- Bill R., Christofori G. The relevance of EMT in breast cancer metastasis: correlation or causality? FEBS Lett. 2015;589:1577–1587. - PubMed
-
- Bukholm I.K., Nesland J.M., Børresen-Dale A.L. Re-expression of E-cadherin, alpha-catenin and beta-catenin, but not of gamma-catenin, in metastatic tissue from breast cancer patients [seecomments] J. Pathol. 2000;190:15–19. - PubMed
-
- Del Pozo Martin Y., Park D., Ramachandran A., Ombrato L., Calvo F., Chakravarty P., Spencer-Dene B., Derzsi S., Hill C.S., Sahai E., Malanchi I. Mesenchymal cancer cell-stroma crosstalk promotes niche activation, epithelial reversion, and metastatic colonization. Cell Rep. 2015;13:2456–2469. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

