Osteoclast-derived exosomal miR-214-3p inhibits osteoblastic bone formation
- PMID: 26947250
- PMCID: PMC4786676
- DOI: 10.1038/ncomms10872
Osteoclast-derived exosomal miR-214-3p inhibits osteoblastic bone formation
Erratum in
-
Author Correction: Osteoclast-derived exosomal miR-214-3p inhibits osteoblastic bone formation.Nat Commun. 2025 Jun 19;16(1):5348. doi: 10.1038/s41467-025-61082-9. Nat Commun. 2025. PMID: 40537508 Free PMC article. No abstract available.
Abstract
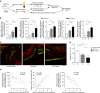
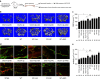
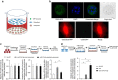
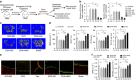
Emerging evidence indicates that osteoclasts direct osteoblastic bone formation. MicroRNAs (miRNAs) have a crucial role in regulating osteoclast and osteoblast function. However, whether miRNAs mediate osteoclast-directed osteoblastic bone formation is mostly unknown. Here, we show that increased osteoclastic miR-214-3p associates with both elevated serum exosomal miR-214-3p and reduced bone formation in elderly women with fractures and in ovariectomized (OVX) mice. Osteoclast-specific miR-214-3p knock-in mice have elevated serum exosomal miR-214-3p and reduced bone formation that is rescued by osteoclast-targeted antagomir-214-3p treatment. We further demonstrate that osteoclast-derived exosomal miR-214-3p is transferred to osteoblasts to inhibit osteoblast activity in vitro and reduce bone formation in vivo. Moreover, osteoclast-targeted miR-214-3p inhibition promotes bone formation in ageing OVX mice. Collectively, our results suggest that osteoclast-derived exosomal miR-214-3p transfers to osteoblasts to inhibit bone formation. Inhibition of miR-214-3p in osteoclasts may be a strategy for treating skeletal disorders involving a reduction in bone formation.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

