Spatial organization of acute myocardial ischemia
- PMID: 26947437
- PMCID: PMC4853261
- DOI: 10.1016/j.jelectrocard.2016.02.014
Spatial organization of acute myocardial ischemia
Abstract
Introduction: Myocardial ischemia is a pathological condition initiated by supply and demand imbalance of the blood to the heart. Previous studies suggest that ischemia originates in the subendocardium, i.e., that nontransmural ischemia is limited to the subendocardium. By contrast, we hypothesized that acute myocardial ischemia is not limited to the subendocardium and sought to document its spatial distribution in an animal preparation. The goal of these experiments was to investigate the spatial organization of ischemia and its relationship to the resulting shifts in ST segment potentials during short episodes of acute ischemia.
Methods: We conducted acute ischemia studies in open-chest canines (N=19) and swines (N=10), which entailed creating carefully controlled ischemia using demand, supply or complete occlusion ischemia protocols and recording intramyocardial and epicardial potentials. Elevation of the potentials at 40% of the ST segment between the J-point and the peak of the T-wave (ST40%) provided the metric for local ischemia. The threshold for ischemic ST segment elevations was defined as two standard deviations away from the baseline values.
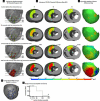
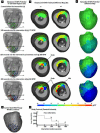
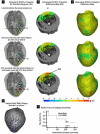
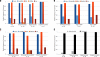
Results: The relative frequency of occurrence of acute ischemia was higher in the subendocardium (78% for canines and 94% for swines) and the mid-wall (87% for canines and 97% for swines) in comparison with the subepicardium (30% for canines and 22% for swines). In addition, acute ischemia was seen arising throughout the myocardium (distributed pattern) in 87% of the canine and 94% of the swine episodes. Alternately, acute ischemia was seen originating only in the subendocardium (subendocardial pattern) in 13% of the canine episodes and 6% of the swine episodes (p<0.05).
Conclusions: Our findings suggest that the spatial distribution of acute ischemia is a complex phenomenon arising throughout the myocardial wall and is not limited to the subendocardium.
Keywords: Acute myocardial ischemia; Spatial organization; Subendocardium; Subepicardium.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures



References
-
- Gorgels A. ST elevation and non-ST elevation acute coronary syndromes: Should the guidelines be changed? Journal of Electrocardiology. 2013;46(4):318–323. - PubMed
-
- O'Rourke R, O'Gara P. Hurst's the Heart - Diagnosis and Management of Patients with CID. McGraw-Hill; New York: 2010. 1em plus 0.5em minus 0.4em.
-
- Reimer K, Jennings R. Myocardial ischemia, hypoxia and mi. In: Fozzard H, et al., editors. The Heart and Cardiovascular System. Raven Press; New York: 1986. pp. 1133–2101. 1em plus 0.5em minus 0.4em.
-
- Kleber A, Janse M, van Capelle F, Durrer D. Mechanism and time course of ST and TQ segment changes during acute regional myocardial ischemia in the pig heart determined by extracellular and intracellular recordings. Circulation Research. 1978;42:603–613. - PubMed
-
- Kubota I, Yamaki M, Shibata T, Ikeno E, Hosoya Y, Tomoike H. Role of ATP-sensitive channel on ECG ST segment elevation during a bout of myocardial ischemia. Circulation. 1993;88:1845–1851. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

