Production of highly bioactive resveratrol analogues pterostilbene and piceatannol in metabolically engineered grapevine cell cultures
- PMID: 26947765
- PMCID: PMC5069453
- DOI: 10.1111/pbi.12539
Production of highly bioactive resveratrol analogues pterostilbene and piceatannol in metabolically engineered grapevine cell cultures
Abstract
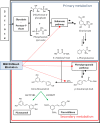
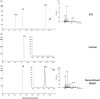
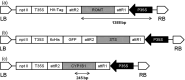
Grapevine stilbenes, particularly trans-resveratrol, have a demonstrated pharmacological activity. Other natural stilbenes derived from resveratrol such as pterostilbene or piceatannol, display higher oral bioavailability and bioactivity than the parent compound, but are far less abundant in natural sources. Thus, to efficiently obtain these bioactive resveratrol derivatives, there is a need to develop new bioproduction systems. Grapevine cell cultures are able to produce large amounts of easily recoverable extracellular resveratrol when elicited with methylated cyclodextrins and methyl jasmonate. We devised this system as an interesting starting point of a metabolic engineering-based strategy to produce resveratrol derivatives using resveratrol-converting enzymes. Constitutive expression of either Vitis vinifera resveratrol O-methyltransferase (VvROMT) or human cytochrome P450 hydroxylase 1B1 (HsCYP1B1) led to pterostilbene or piceatannol, respectively, after the engineered cell cultures were treated with the aforementioned elicitors. Functionality of both gene products was first assessed in planta by Nicotiana benthamiana agroinfiltration assays, in which tobacco cells transiently expressed stilbene synthase and VvROMT or HsCYP1B1. Grapevine cell cultures transformed with VvROMT produced pterostilbene, which was detected in both intra- and extracellular compartments, at a level of micrograms per litre. Grapevine cell cultures transformed with HsCYP1B1 produced about 20 mg/L culture of piceatannol, displaying a sevenfold increase in relation to wild-type cultures, and reaching an extracellular distribution of up to 45% of total production. The results obtained demonstrate the feasibility of this novel system for the bioproduction of natural and more bioactive resveratrol derivatives and suggest new ways for the improvement of production yields.
Keywords: Vitis vinifera; grapevine cell culture; metabolic engineering; piceatannol; pterostilbene; resveratrol.
© 2016 The Authors. Plant Biotechnology Journal published by Society for Experimental Biology and The Association of Applied Biologists and John Wiley & Sons Ltd.
Figures






References
-
- Aggarwal, B.B. , Bhardwaj, A. , Aggarwal, R.S. , Seeram, N.P. , Shishodia, S. and Takada, Y. (2004) Role of resveratrol in prevention and therapy of cancer: preclinical and clinical studies. Anticancer Res. 24, 2783–2840. - PubMed
-
- Almagro, L. , Belchí‐Navarro, S. , Sabater‐Jara, A.B. , Vera‐Urbina, J.C. , Selles‐Marchart, S. , Bru, R. and Pedreño, M.A. (2013) Bioproduction of trans‐resveratrol from grapevine cell cultures. In Handbook of Natural Products, ( Ramawat, K.G. and Merillon, J.M. , eds), pp. 1683–1713. Heidelberg: Springer.
-
- Asensi, M. , Medina, I. , Ortega, A. , Carretero, J. , Baño, M.C. , Obrador, E. and Estrela, J.M. (2002) Inhibition of cancer growth by resveratrol is related to its low bioavailability. Free Radic. Biol. Med. 33, 387–398. - PubMed
-
- Baur, J.A. and Sinclair, D.A. (2006) Therapeutic potential of resveratrol: the in vivo evidence. Nat. Rev. Drug Discov. 5, 493–506. - PubMed
-
- Bavaresco, L. , Fregoni, M. , Trevisan, M. , Mattivi, F. , Vrhovsek, U. and Falchetti, R. (2002) The occurrence of the stilbene piceatannol in grapes. Vitis, 41, 133–136.
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

