Current Advances in Developing Inhibitors of Bacterial Multidrug Efflux Pumps
- PMID: 26947776
- PMCID: PMC5425656
- DOI: 10.2174/0929867323666160304150522
Current Advances in Developing Inhibitors of Bacterial Multidrug Efflux Pumps
Abstract
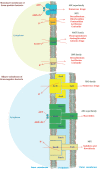
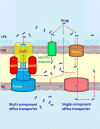
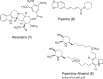
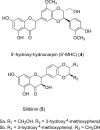
Antimicrobial resistance represents a significant challenge to future healthcare provision. An acronym ESKAPEE has been derived from the names of the organisms recognised as the major threats although there are a number of other organisms, notably Neisseria gonorrhoeae, that have become equally challenging to treat in the clinic. These pathogens are characterised by the ability to rapidly develop and/or acquire resistance mechanisms in response to exposure to different antimicrobial agents. A key part of the armoury of these pathogens is a series of efflux pumps, which effectively exclude or reduce the intracellular concentration of a large number of antibiotics, making the pathogens significantly more resistant. These efflux pumps are the topic of considerable interest, both from the perspective of basic understanding of efflux pump function, and its role in drug resistance but also as targets for the development of novel adjunct therapies. The necessity to overcome antimicrobial resistance has encouraged investigations into the characterisation of resistance-modifying efflux pump inhibitors to block the mechanisms of drug extrusion, thereby restoring antibacterial susceptibility and returning existing antibiotics into the clinic. A greater understanding of drug recognition and transport by multidrug efflux pumps is needed to develop clinically useful inhibitors, given the breadth of molecules that can be effluxed by these systems. This review discusses different bacterial EPIs originating from both natural source and chemical synthesis and examines the challenges to designing successful EPIs that can be useful against multidrug resistant bacteria.
Figures

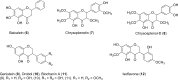
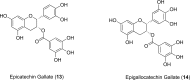
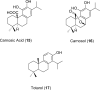
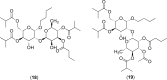


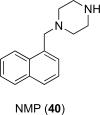

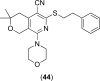
References
-
- Ruef C. Epidemiology and clinical impact of glycopeptide resistance in Staphylococcus aureus. Infection. 2004;32(6):315–327. - PubMed
-
- Rice L.B. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: no ESKAPE. J. Infect. Dis. 2008;197(8):1079–1081. - PubMed
-
- Piddock L.J. The crisis of no new antibiotics—what is the way forward? Lancet Infect. Dis. 2012;12(3):249–253. - PubMed
-
- Collins A.S. 2008. Preventive Health Care-Associated Infections.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

