Tissue factor deficiency increases alveolar hemorrhage and death in influenza A virus-infected mice
- PMID: 26947929
- PMCID: PMC5892427
- DOI: 10.1111/jth.13307
Tissue factor deficiency increases alveolar hemorrhage and death in influenza A virus-infected mice
Erratum in
-
Corrigendum.J Thromb Haemost. 2016 Dec;14(12):2565. doi: 10.1111/jth.13521. Epub 2016 Dec 1. J Thromb Haemost. 2016. PMID: 28026156 No abstract available.
Abstract
Essentials H1N1 Influenza A virus (IAV) infection is a hemostatic challenge for the lung. Tissue factor (TF) on lung epithelial cells maintains lung hemostasis after IAV infection. Reduced TF-dependent activation of coagulation leads to alveolar hemorrhage. Anticoagulation might increase the risk for hemorrhages into the lung during severe IAV infection.
Summary: Background Influenza A virus (IAV) infection is a common respiratory tract infection that causes considerable morbidity and mortality worldwide. Objective To investigate the effect of genetic deficiency of tissue factor (TF) in a mouse model of IAV infection. Methods Wild-type mice, low-TF (LTF) mice and mice with the TF gene deleted in different cell types were infected with a mouse-adapted A/Puerto Rico/8/34 H1N1 strain of IAV. TF expression was measured in the lungs, and bronchoalveolar lavage fluid (BALF) was collected to measure extracellular vesicle TF, activation of coagulation, alveolar hemorrhage, and inflammation. Results IAV infection of wild-type mice increased lung TF expression, activation of coagulation and inflammation in BALF, but also led to alveolar hemorrhage. LTF mice and mice with selective deficiency of TF in lung epithelial cells had low basal levels of TF and failed to increase TF expression after infection; these two strains of mice had more alveolar hemorrhage and death than controls. In contrast, deletion of TF in either myeloid cells or endothelial cells and hematopoietic cells did not increase alveolar hemorrhage or death after IAV infection. These results indicate that TF expression in the lung, particularly in epithelial cells, is required to maintain alveolar hemostasis after IAV infection. Conclusion Our study indicates that TF-dependent activation of coagulation is required to limit alveolar hemorrhage and death after IAV infection.
Keywords: hemorrhage; hemostasis; influenza A virus; pneumonia; tissue factor.
© 2016 International Society on Thrombosis and Haemostasis.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures

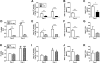
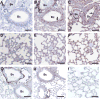



References
-
- Kroger AT, Atkinson WL, Marcuse EK, Pickering LK Advisory Committee on Immunization Practices Centers for Disease C, Prevention. General recommendations on immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2006;55:1–48. - PubMed
-
- Geisbert TW, Young HA, Jahrling PB, Davis KJ, Kagan E, Hensley LE. Mechanisms underlying coagulation abnormalities in ebola hemorrhagic fever: overexpression of tissue factor in primate monocytes/macrophages is a key event. J Infect Dis. 2003;188:1618–29. - PubMed
-
- Funderburg NT, Mayne E, Sieg SF, Asaad R, Jiang W, Kalinowska M, Luciano AA, Stevens W, Rodriguez B, Brenchley JM, Douek DC, Lederman MM. Increased tissue factor expression on circulating monocytes in chronic HIV infection: relationship to in vivo coagulation and immune activation. Blood. 2010;115:161–7. - PMC - PubMed
-
- Huerta-Zepeda A, Cabello-Gutierrez C, Cime-Castillo J, Monroy-Martinez V, Manjarrez-Zavala ME, Gutierrez-Rodriguez M, Izaguirre R, Ruiz-Ordaz BH. Crosstalk between coagulation and inflammation during Dengue virus infection. Thromb Haemost. 2008;99:936–43. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

